Structural Biological Materials: Difference between revisions
KuroKitten (talk | contribs) |
No edit summary |
||
| (26 intermediate revisions by 3 users not shown) | |||
| Line 5: | Line 5: | ||
Rather than simply take the approach of listing options, this page will aim to explore the general categories of structural biological materials - both real and theoretical - and explain how their particular properties play into how they're used in living creatures. | Rather than simply take the approach of listing options, this page will aim to explore the general categories of structural biological materials - both real and theoretical - and explain how their particular properties play into how they're used in living creatures. | ||
==Polysaccharides | ==Polysaccharides== | ||
Polysaccharides are perhaps the most widespread - and simplest to evolve - structural materials we find in living creatures. Saccharides, especially glucose, are universally used as energy storage in Earth life, and it's likely that alien life - at least, that which shares our basic biochemistry - will do the same. It therefore isn't much of a step to derive your structural materials from the stuff you already use for energy storage! | Polysaccharides are perhaps the most widespread - and simplest to evolve - structural materials we find in living creatures. Saccharides, especially glucose, are universally used as energy storage in Earth life, and it's likely that alien life - at least, that which shares our basic biochemistry - will do the same. It therefore isn't much of a step to derive your structural materials from the stuff you already use for energy storage! | ||
Even though monosaccharides are typically hydrophilic, and readily dissolve in water, most polysaccharides are, in fact, quite hydrophobic and insoluble. This makes them quite well-suited for assembly-on-the-spot - the component monosaccharides can be dissolved in the cellular medium for transport, and will precipate out of the water as they are assembled into polysaccharides. | |||
One defining element of polysaccharides, on the chemical level, is the multiple free hydroxyl groups on each monomer of the chain. Through a variety of processes, these can be replaced by other functional groups, altering the properties of the polysaccharide; they may also serve as useful bonding sites for other structural molecules, such as peptides and polyphenols. This chemical versatility is another reason why they are so ubuiquitous in Earth life - they excel at a wide variety of structural purposes, and make a very good "foundation" for other structural biological materials to build on in composite materials. | |||
===Cellulose, and other simple linear polysaccharides=== | ===Cellulose, and other simple linear polysaccharides=== | ||
[https://en.wikipedia.org/wiki/Cellulose Cellulose] is the simplest and most widespread polysaccharide that sees structural use on Earth, and is likely to be so on alien worlds as well. It's a simple linear chain of β(1→4) linked units of d-glucose - the same molecule nearly all life on Earth uses to store energy. Aliens might use ʟ-glucose instead, but this should have identical mechanical properties. | |||
Contrary to popular belief, cellulose is actually quite flexible, when alone. The rigidity of plant cells - famously with cell walls made out of cellulose - is provided by other materials bonded with cellulose, such as [[Structural Biological Materials#Polyphenols (lignin, tannin, etc.)|lignin]], along with the hydrostatic pressure provided by the water within the cell. | Contrary to popular belief, cellulose is actually quite flexible, when alone. The rigidity of plant cells - famously with cell walls made out of cellulose - is provided by other materials bonded with cellulose, such as [[Structural Biological Materials#Polyphenols (lignin, tannin, etc.)|lignin]], along with the hydrostatic pressure provided by the water within the cell. | ||
A number of Earth organisms are demonstrated to use mannose, or other simple sugars, in the place of glucose, forming structural polysaccharides such as | A number of Earth organisms are demonstrated to use mannose, or other simple sugars, in the place of glucose, forming structural polysaccharides such as [https://en.wikipedia.org/wiki/Mannan_(polysaccharide) mannan]. Their properties are similar to cellulose - though, how they may bond to other polymers might differ, as we'll discuss later with glucomannans. | ||
Some other organisms, such as oomycetes, use other β-glucans - that is, linear chains of glucose - which have different linkages (such as the β(1→3) linkages of chrysolaminarin) as structural materials. In bulk, their mechanical properties are fairly similar. | Some other organisms, such as oomycetes, use other β-glucans - that is, linear chains of glucose - which have different linkages (such as the β(1→3) linkages of chrysolaminarin) as structural materials. In bulk, their mechanical properties are fairly similar. | ||
[[File:Cellulose.png|thumb | [[File:Cellulose.png|thumb|none|The structural formula of cellulose.]] | ||
===Hemicelluloses - heterogenous simple polysaccharides=== | |||
In plants, cellulose is not found alone - it is typically accompanied by filaments of [https://en.wikipedia.org/wiki/Hemicellulose hemicellulose], otherwise known as '''polyose''', a group of highly heterogenous polysaccharides composed of a semi-random mix of miscellaneous saccharide molecules - predominately arabinose and xylose. The heterogeneity of these fibers inhibits the formation of crystalline structures the way cellulose might form, rendering the result relatively amorphous and weak: if isolated, hemicelluloses would have a gum- or gel-like texture. While that may seem undesirable in a structural material, hemicelluloses perform important functions in plants - in particular, cross-linking cellulose fibers together, adding additional strength and toughness to the resulting matrix. | |||
[[File:Xylan_hardwood.png|thumb|none|Structural formula of a xylan hemicellulose found in a hardwood species.]] | |||
===Chitin, and other poly-amino-sugars=== | ===Chitin, and other poly-amino-sugars=== | ||
[https://en.wikipedia.org/wiki/Chitin Chitin] is the second most widespread structural polysaccharide on Earth, and for good reason. Like cellulose, it is a linear chain of single monomers. Rather than being made of simple glucose, however, it is composed of [https://en.wikipedia.org/wiki/N-Acetylglucosamine ''N''-acetylglucosamine] - an amino-sugar of glucose where one of the hydroxyl groups is replaced by an acetylamino moiety. This acetylamino moiety forms stronger hydrogen bonds with adjacent polymers than the hydroxyl group would, rendering chitin much stronger and slightly more rigid than cellulose - though, it still remains plenty flexible, without other components to rigidify it. | |||
''N''-acetylmannosamine and ''N''-acetylgalactosamine, derivatives of mannose and galactose, respectively, also exist in nature - to my knowledge, they are not used to form polymers like chitin, but an alien organism may do so! | ''N''-acetylmannosamine and ''N''-acetylgalactosamine, derivatives of mannose and galactose, respectively, also exist in nature - to my knowledge, they are not used to form polymers like chitin, but an alien organism may do so! | ||
===Algin, and other polyuronic acids=== | [[File:Chitin Highlighted.png|thumb|none|Structural formula of chitin. Notice the acetylamino moieties, here highlighted in red.]] | ||
===Hydrophilic polysaccharides=== | |||
Though most polysaccharides are hydrophobic, this does not apply to all. Many are possessed of an overall negative charge and/or are internally polar, allowing them to become quite hydrophilic. As hydrophilic molecules, they will readily dissolve in water - a characteristic exploited in certain biological lubricants, such as the fluid that fills the space between joints in vertebrates, which consists of relatively short chains of hydrophilic polysaccharides dissolved in water (when you pop your knuckles - or other joints - what you're doing is popping bubbles in this fluid). | |||
However, though these polysaccharides dissolve in water, this ''does not break the bonds of the polymers''. This is a property of water-soluble polymers in general - though they may "dissolve", they do not break apart. This means relatively long polymers, especially those with extensive cross-linkages between them, will maintain their structural form when hydrated - instead, the water infiltrates the polymer structure, causing it to swell into a sort of hydrogel or gum, which may be stiffer or softer depending on how closely interlinked the polymer chains within it are. Water can also pass through the polysaccharide gel, at a rate which is, again, dependent on the exact structure of the gel - which may assist organisms, particularly unicellular or colonial ones, in osmoregulation. This structural usage of hydrophilic polysaccharides is obviously better suited for aquatic biota, which do not have to worry about their polysaccharide hydrogels drying out - but can still be quite useful in the interior structures of terrestrial biota. | |||
====Algin, and other polyuronic acids==== | |||
[https://en.wikipedia.org/wiki/Alginic_acid Algin], or alginic acid, a polyuronic acid, is a primary structural component of the cell walls of brown algae, such as kelp. It is a polymer of two uronic acids in alternating sequence - β-D-mannuronate and α-L-guluronate. Uronic acids are derivatives of saccharides where the hydroxyl group opposite the carbonyl has been oxidized to a carboxyl group, making them a kind of carboxylic acid. This makes polyuronic acids acidic (as implied by the name) - which in turn makes them hydrophilic and water-soluble. | |||
The hydrophilic nature of algin is responsible for the gummy, gel-like texture/structure of the brown algae. | |||
The carboxyl group replacement that defines the uronic acids very rarely co-occurs with replacements of other hydroxyls on the same monomer with other functional groups, for reasons I don't personally understand. For example, I know of only one amino uronic acid - [https://en.wikipedia.org/wiki/N-Acetyltalosaminuronic_acid ''N''-Acetyltalosaminuronic acid]. | |||
[[File:AlginHighlighted.png|thumb|none|Structural formula of algin. Notice the carboxyl groups, highlighted in blue.]] | |||
====Sulfated polysaccharides==== | |||
Sulfated polysaccharides are common throughout Earth life, and should be expected to be common among alien organisms with broadly similar biochemistry. In sulfated polysaccharides, one or more free hydroxyl groups are replaced by a sulfate ion; in some cases, sulfates occur only once every several monomers, while in others, nearly every single hydroxyl group in the entire chain is replaced by a sulfate. | |||
Like polyuronic acids, sulfated polysaccharides are hydrophilic, due to the negative charge of the sulfate ion. However, they have a range of properties which distinguishes them profoundly from their uronic acid equivalents. | |||
First, whereas the carboxyl groups that define the uronic acids rarely co-occur with other hydroxyl-replacements on the same monomer, sulfation ''frequently'' occurs with other functional group replacements - particularly, sulfated amino sugars, where one or more of the remaining hydroxyl groups (after the addition of the amine) has been sulfated, are quite common in nature, allowing a single monomer to bear the characteristics brought by both sulfation and other derivative processes. This extends to, as previously mentioned, molecules where each monomer has had ''all'' free hydroxyl groups sulfated, resulting in a much greater negative charge (and thus, greater hydrophilicity) than in uronic acids. | |||
Second, sulfated polysaccharides are quite good at binding and precipitating metal ions, such as calcium or sodium, out of solution. Relatively short sulfated polysaccharides, therefore, often play a non-structural role in regulating the transport of ions throughout an organism; and sulfated polysaccharides in the outer surfaces of aquatic organisms, such as in the cell walls of many algae, may assist in the capture of necessary ions from the water. However, this also lends itself to a structural purpose - sulfated polysaccharides appear to play a role in biomineralization in some clades, such as in the coralline algaes, precipitating metal ions out of solution to form hard, mineralized coatings on the organism's exterior. | |||
===Disaccharide polysaccharides=== | ===Disaccharide polysaccharides=== | ||
As demonstrated by algin, above, structural polysaccharides, rather than consisting of a homogenous chain of a single monosaccharide molecule, are instead often composed of two distinct monosaccharides in alternating sequence. This allows the copolymer to have some of the properties offered by both monosaccharides. | As demonstrated by algin, above, many structural polysaccharides, rather than consisting of a homogenous chain of a single monosaccharide molecule, are instead often composed of two distinct monosaccharides in alternating sequence. This allows the copolymer to have some of the properties offered by both monosaccharides. | ||
====Glycosaminoglycans==== | |||
[https://en.wikipedia.org/wiki/Glycosaminoglycan Glycosaminoglycans], also known as '''mucopolysaccharides''', are a common form of alternating polysaccharide, consisting of alternating units of a uronic acid and an amino sugar, the latter of which may be sulfated (as in [https://en.wikipedia.org/wiki/Heparin heparin]) or not (as in [https://en.wikipedia.org/wiki/Hyaluronic_acid hyaluronan]). These polysaccharides are ''very'' polar, and attract water very strongly as a result, while also self-adhering quite well. | |||
Glycosaminoglycans fulfill a variety of important functions, particularly in animals. Since diffusion through them is possible, but quite slowly, they help slow and regulate the intercellular diffusion of substances like ions and proteins; since they can adhere quite well, they serve as an adhesive binding different cells together; and since they attract water so well, they are used to assist in the retention of moisture where watery surfaces must be exposed, such as in the eyes of vertebrates. | |||
====Peptidoglycan==== | |||
[https://en.wikipedia.org/wiki/Peptidoglycan Peptidoglycan], also known as murein, is the primary structural component of bacterial cell walls. It is an alternating copolymer of ''N''-acetylglucosamine (as found in chitin) and ''N''-acetylmuramic acid - a complex monosaccharide where a lactic acid moiety has replaced yet another hydroxyl group on ''N''-acetylglucosamine. Like in chitin, the acetylamino moieties serve to assist in the formation of crystalline structures through strong hydrogen bonds; the addition of the lactic acid to every other monomer in the chain, however, serves as a strong binding site for short oligopeptides (consisting of three to five amino acids) which bind to a different polysaccharide chain at each end, forming a regular mesh that resembles a chain-link fence. | |||
There | The resulting structure is very hard to break apart chemically, and strong enough to maintain the regular shape of cells observed in most bacteria. There is no particular reason, in fact, why it is not found in eukaryotes or archaeans, other than these two groups simply not having evolutionarily stumbled upon it - peptidoglycan only evolved once. So, complex alien life might use it! | ||
===Branching polysaccharides=== | ===Branching polysaccharides=== | ||
As seen above, almost all polysaccharides used for structural purposes in living organisms are linear chains, though branching polysaccharides are commonly used for energy storage. However, some branching polysaccharides do, in fact, see structural use - such as the '''glucomannans''' found in some yeasts and plants. Glucomannans consist of a linear chain of mannose units (called a mannan), with short glucose branches. Unlike linear polysaccharides, branched polysaccharides resist forming neat, crystalline structures (as the branches interfere with hydrogen-bond formation between polymers), and thus are able to slide past each other, forming a rubbery, hydrophilic solid. | As seen above, almost all polysaccharides used for structural purposes in living organisms are linear chains, though branching polysaccharides are commonly used for energy storage. However, some branching polysaccharides do, in fact, see structural use - such as the '''glucomannans''' found in some yeasts and plants. Glucomannans consist of a linear chain of mannose units (called a mannan), with short glucose branches. Unlike linear polysaccharides, branched polysaccharides resist forming neat, crystalline structures (as the branches interfere with hydrogen-bond formation between polymers), and thus are able to slide past each other, forming a rubbery, hydrophilic solid, or even a viscous liquid (when hydrated). | ||
==Polyphenols (lignin, tannin, etc.)== | ==Polyphenols (lignin, tannin, etc.)== | ||
| Line 64: | Line 99: | ||
Some - not all - polyphenols, such as lignins, are exceptionally resistant to bio-degradation, making them very difficult for other living things to break down and digest - and this extends to the other polymers they bind to. This makes them very good at protecting from penetration by parasites or pathogens, and limits the possible predators of organisms that use polyphenol cross-linking in their structures. | Some - not all - polyphenols, such as lignins, are exceptionally resistant to bio-degradation, making them very difficult for other living things to break down and digest - and this extends to the other polymers they bind to. This makes them very good at protecting from penetration by parasites or pathogens, and limits the possible predators of organisms that use polyphenol cross-linking in their structures. | ||
===Affinity for saccharides=== | |||
Polyphenols have something of a special affinity for saccharides, and will not only readily bond with them, but also often include one or more saccharide molecules in their structure - such as in tannic acid, a polyphenol composed of phenol branches stemming from a glucose molecule at its center. | |||
{|style="margin: 0 auto;" | |||
| [[File:Phenol2.png|thumb|upright=0.59|Structural formula of phenol, the simplest phenol.]] | |||
| [[File:TannicAcid.png|thumb|Structural formula of tannic acid, a polyphenol. Notice that it is built with a monosaccharide - glucose, specifically - at its center.]] | |||
| [[File:Lignin.png|thumb|none|Idealized structure of lignin from a softwood. In reality, lignin molecules are highly heterogenous, and do not have a consistent structure.]] | |||
|} | |||
==Cross-linking proteins (keratins, sclerotins, etc.)== | ==Cross-linking proteins (collagens, keratins, sclerotins, etc.)== | ||
Proteins - perhaps unsurprisingly, given their ubiquity - are common components of structural biological materials, specifically in the form of cross-linking proteins like keratins or sclerotins, which bond to each other at many different sites, allowing for not only toughness and rigidity but a ''tunable'' degree of both - via controlling the frequency of cross-linkages, materials can smoothly vary in rigidity and hardness, often forming distinct gradients in biological structures (such as arthropod cuticle becoming more flexible around the joints). | Proteins - perhaps unsurprisingly, given their ubiquity - are common components of structural biological materials, specifically in the form of cross-linking proteins like keratins or sclerotins, which bond to each other at many different sites, allowing for not only toughness and rigidity but a ''tunable'' degree of both - via controlling the frequency of cross-linkages, materials can smoothly vary in rigidity and hardness, often forming distinct gradients in biological structures (such as arthropod cuticle becoming more flexible around the joints). | ||
Most cross-linking structural proteins tend to be brown to black in color, though are not particularly opaque, and some can be outright colorless. Their versatility also lends themselves to the creation of complex microstructures resulting in vivid structural colorations, and tissue that includes them often also plays host to a variety of pigments that add their own colors to the material. | |||
===Collagens=== | |||
Collagens are the primary family of structural proteins found in all metazoans, though they are most prominent in the deuterostomes - in protostomes, many of the functions typically performed by collagens are instead performed by chitin. In mammals, collagens make up 25-35% of all proteins in the body! They make up the connective tissue, while also adding strength to other tissues (such as blood vessels) throughout the body, and also providing the matrix on which hydroxyapatite is deposited to form bone and cartilage in vertebrates. | |||
Collagen consists of a left-handed triple helix of elongated peptide chains. About half of its amino acid content is glycine and proline alone, which appear to play a role in binding the three components of the helix together. In addition, sites throughout the chain are saturated in hydroxylysine - a hydroxylated derivative of the amino acid lysine. It is by these hydroxylysine sites that collagen helixes are cross-linked - via aldol condensation, strong covalent bonds are formed through conjugated enone moieties between the hydroxylysine sites on adjacent collagen helixes. This binds the adjacent helixes together very strongly, while still allowing hydrolysis of the linkages when they must be split apart, unlike certain other structural proteins. | |||
Unlike most structural proteins, collagen appears colorless, or sometimes white. It is also extremely flexible - collagenous tissue must rely on other components to add hardness and rigidity, if necessary. | |||
===Keratins=== | ===Keratins=== | ||
| Line 85: | Line 137: | ||
Sclerotization is effectively non-reversible - cross-linked sclerotins being extremely hard to break apart - and thus organisms must shed sclerotinized parts if they need to change them, hence the necessity of molting in arthropods. | Sclerotization is effectively non-reversible - cross-linked sclerotins being extremely hard to break apart - and thus organisms must shed sclerotinized parts if they need to change them, hence the necessity of molting in arthropods. | ||
Some annelids, such as the intimidatingly-named bloodworm, have special sclerotins which bind metal ions, such as copper and iron, prior to undergoing sclerotization. This results in unique metal-composite structures which are exceptionally hard and strong<ref name="BloodwormJaw">[https://www.cell.com/matter/fulltext/S2590-2385(22)00153-9]</ref>. | Some annelids, such as the intimidatingly-named bloodworm, have special sclerotins which bind metal ions, such as copper and iron, prior to undergoing sclerotization. This results in unique metal-composite structures which are exceptionally hard and strong.<ref name="BloodwormJaw">[https://www.cell.com/matter/fulltext/S2590-2385(22)00153-9]</ref> | ||
===Other cross-linking proteins=== | |||
Other cross-linking proteins are found in other clades, though none as archetypical and widespread as keratins and sclerotins. | |||
For example, cephalopod mollusks possess their own particular cross-linking proteins that add hardness and rigidity to their beaks, operating in a two-tiered system - they are, unfortunately, absent a catchy name like keratins and sclerotins, and are instead simply referred to as '''chitin-binding beak proteins (CBPs)''' and '''histidine-rich beak proteins (HBPs)'''. CBPs simply bind to multiple chitin chains at both ends, forming cross-links between them. HBPs add a second tier to this matrix, and bond to both CBPs and each other, forming an additional set of cross-links to add even more strength, hardness, and rigidity to the result.<ref name="CephalopodBeak">[https://www.nature.com/articles/nchembio.1833]</ref> | |||
Alien organisms may be expected to exhibit yet more variations on the common motif of proteins cross-linked together, unseen on Earth - instead of directly using one found on Earth, it may be better to simply specify a, say, "keratin-like protein"! | |||
==Biominerals== | |||
While all the structural materials discussed previously - and those which will be discussed after - are polymers, mineral crystals also play a major role in structural biological materials. Biominerals excel at adding hardness, strength, and rigidity to biological structures; only a handful of organic polymers are capable of matching even the softest biominerals in hardness and rigidity. | |||
For obvious reasons, tissue that is heavily biomineralized cannot be flexible - although, semi-flexible partially-biomineralized tissue, such as cartilage in vertebrate, does exist in nature. | |||
In addition to performing structural functions, biomineralized tissue also often functions as a reservoir of biologically-essential chemicals, such as metal ions, phosphate, and sulfate. In fact, many biomineralized structures in modern Earth organisms are believed to have evolved from what were initially purely storage deposits - for example, the bones of vertebrates are believed to be derived from what was initially the mere deposition of calcium and phosphate for storage. | |||
All biomineralization processes found in nature (at least, that I am aware of) involve the creation of ''insoluble'' crystals from ''soluble'' components. The necessity of this particular combination of characteristics - soluble components, insoluble products - is obvious when you consider the challenges involved in creating them: chemicals must be soluble in water (or whatever alternative solvent) to be acquired from the environment and transported through an organism's tissues; and upon forming their insoluble products, they immediately precipitate out of solution, forming hard, insoluble crystals, and thus useful structural materials. This means that '''components that are insoluble cannot be acquired and transported, and products that are soluble cannot be made into useful structural materials.''' This renders certain sci-fi go-tos - such as giving an alien organism bones of titanium - implausible (as titanium is insoluble in water in all forms). However, there is still plenty of room to explore hypothetical biominerals, as will soon be demonstrated. | |||
While most biominerals are salts - specifically, salts of metal cations and oxyanions - we will first discuss the exceptions to that rule. | |||
===Silicates=== | |||
[[File:Diatoms through the microscope.jpg|thumb|right|200px|An assortment of unicellular diatoms under light microscopy.]] | |||
[[File:Diatoms Egge and Aksnes 1992 plot.png|thumb|right|200px|A chart showing diatoms as a proportion of algal populations, relative to silicate concentration. It shows the extreme dependency of silicate-biomineralizing organisms on silicic acid concentrations.]] | |||
'''Silicates''', otherwise known as common glasses (literally, like the kind in your window), are perhaps the most common and widespread kind of structural biominerals on Earth, having evolved independently countless times throughout Earth's tree of life. This may seem surprising, given that you probably know of very few living things that are made out of glass - and that's because almost all organisms that use silica as a structural biomineral are unicellular. While clades that use silica as a ''minor'' structural component are common among multicellular life here on Earth - such as the horsetail ferns - there is only one that is known to use it as the primary mineral component of their structural materials: the Hexactinellid sponges, otherwise known as the '''glass sponges.''' | |||
Silicates are also one of the few biominerals thare are not typically found in the form of a salt - instead being typically formed of silica (SiO₂) in its various crystalline forms. Silica is extremely chemically inert, and therefore virtually useless to any living organism for biochemical processes - therefore, unlike many other biominerals, it is ''solely'' a structural material, and not a reservoir of essential nutrients. On the other hand, this non-reactivity makes it exceptional for structural purposes, and it is one of the hardest and strongest biominerals found in nature! | |||
One may find the apparent ubiquity of biogenic silica confusing, in context of previous remarks on the necessity that biominerals be the insoluble products of soluble components, as silicates are not usually thought to be soluble - the truth, however, is that silicates are ''very weakly'' soluble, depending on their crystalline structure. While, ordinarily, such weak solubility would be insufficient to supply enough of the compound to a planet's oceans, the key lies in that ''silica makes up the vast majority of the Earth's crust'', and can be expected to do the same on alien worlds of similar composition to Earth; thus, the relatively small ''portion'' of silica that dissolves is actually quite gigantic, in absolute terms! And once this dissolved silica has been re-formed into crystals, it does not readily dissolve again - thus fulfilling that same criterion. | |||
Silicate biomineralization is typically performed by precipitating silicic acid - the dissolved form of silica - out of water, onto a matrix of specialized biopolymer filaments. This not only results in a very strong structural material, it is also, energetically, exceptionally cheap for the organism in question - organisms that use silica as a structural material spend only a fraction of the energy forming silicate structures as other biominerals require. | |||
This, however, does mean that an organism's ability to build biological structures out of silica is strictly limited by the availability of silicic acid in its environment - and given how silicic acid enters the environment in the first place, this quantity is quite limiting indeed. In fact, competition over silicic acid in the biosphere of Earth is quite fierce: Hexactinellid sponges once were one of the dominant clades of filter-feeder throughout the water column, forming reefs comparable to today's corals, but are today limited entirely to deep water environments - by the Cenozoic period, the diatoms had become ecologically ascendant (though they are believed to have first appeared in the Triassic, they did not become ecologically dominant until much later), and nearly monopolized the supply of silicic acid in the ocean's photic zone, making it impossible for the shallow-water glass sponges to obtain enough to build their spicules. The implications of this for alien ecosystems are profound - in order for silica to be more widely-used in an alien ecosystem than it is on Earth, something will have to dramatically increase the availability of silicic acid in the planet's waters. As an example - adding ammonia or methanol to water substantially increases the solubility of silica, but not enough to make silica less useful as a biomineral; thus, the subsurface oceans of many ice worlds (provided the lower surface is silica, and not another layer of ice), thought to consist of eutectic mixtures of water and ammonia, may be replete with silica-biomineralizing life! | |||
As a biomineral, silica is essentially always found in the form of [https://en.wikipedia.org/wiki/Opal opal], the hydrated, amorphous form of silica. This is, perhaps unfortunately, ''not'' typically what we would identify as ''precious'' opal - the gemstone known for its vibrant plays of color - but common opal, weakly translucent and milky-gray in color. Given that precious opal is merely a ''particular arrangement'' of hydrated silica, however, it is entirely plausible that an alien organism might evolve surface structures of precious opal as a form of display! | |||
===Metal Oxides and Sulfides=== | |||
A few organisms, in need of exceptionally hard and tough biominerals, have evolved the use of transition metal oxides and sulfides - particularly the oxides and sulfides of iron. Unlike metal salts, they do not make particularly good reservoirs of essential metals for other biological purposes - they are quite strongly bound, and thus, like silicates, are almost solely used as structural materials. However, they do also, potentially, have one other interesting application - many of these minerals (particularly the iron-oxide [https://en.wikipedia.org/wiki/Magnetite magnetite] and the iron-sulfide [https://en.wikipedia.org/wiki/Greigite greigite]) are ferromagnetic, and thus many organisms use them as components of [https://en.wikipedia.org/wiki/Magnetosome magnetosomes] - organelles for sensing geomagnetic fields, and a kind of biological compass. | |||
It is not particularly common for these biominerals to occur as major structural components throughout an organism's body - rather, most cases involve, quite specifically, ''teeth''. Metal oxides and sulfides are generally energetically and nutritionally expensive to form; therefore, they tend to be used for specialized purposes, with more common biominerals being used elsewhere. As an example, [https://en.wikipedia.org/wiki/Chiton chitons] and [https://en.wikipedia.org/wiki/Limpet limpets] both have independently-evolved iron oxide teeth ([https://en.wikipedia.org/wiki/Magnetite magnetite] in the former case; [https://en.wikipedia.org/wiki/Goethite goethite] in the latter), which they use to scrape algae and other organisms from hard rocks on the seabed (their teeth being so exceptional at this that they are significant contributors to erosion) - however, both possess defensive shells of the rather more ordinary calcium carbonate, instead. | |||
The exception, of course, occurs where metals to oxidize - and oxygen or sulfur to react them with - are easily found, such as around hydrothermal vents, which spew large amounts of sulfur and iron into the surrounding ocean. It is here that the snail [https://en.wikipedia.org/wiki/Scaly-foot_gastropod Chrysomalion squamiferum], the scaly-foot snail, is found - with its shell and protective scales made of iron sulfides. | |||
These oxides and sulfides of metals are typically formed by reacting oxygen or sulfur with metal ions inside of tissues, which then preciptates out as the corresponding metal oxide/sulfide. | |||
===Salts=== | |||
As previously mentioned, the most common biominerals on Earth are mineral salts - particularly, salts of a transition or alkaline metal cation (such as calcium, magnesium, or iron) with an oxyanion (such as carbonate, phosphate, or sulfate). | |||
As with other biominerals, the anion and the cation must be soluble in water; however, the resulting salt crystal must be insoluble, and thus able to precipitate out of solution. Unlike with silicates, which are always very weakly soluble, and with metal oxides and sulfides, which go from very soluble to completely insoluble, the gap between the solubilities of salts and their component ions is typically rather small; changing the pH of the solution may render insoluble salts soluble, or soluble salts insoluble, changing which make for workable biominerals. | |||
====Cations==== | |||
While there are quite a large number of metals, both alkaline and transitional, that can potentially form salts with oxyanions, only a handful make for plausible components of biominerals. Most are simply too rare to be used in bulk for structural materials in biology. Many of those that remain are biochemically inert - particularly those that are completely insoluble in water, such as aluminum and titanium (sorry, SpecEvo writers who like giving their creatures titanium bones - it doesn't work! Titanium is biochemically inert). And some that are both common and very biochemically active, such as sodium and potassium, only seem to form salts that are soluble. | |||
While we typically characterize biominerals as being composed of one cation in particular (e.g. we speak of our bones as being made from calcium), in reality, virtually all biomineral salts actually contain a mix of cations, though one might predominate - the bones of vertebrates actually contain significant amounts of magnesium and manganese in addition to calcium, for instance. | |||
Notably, it is typically the cations, not the anions, that typically determines the color of mineral salts - in the absence of other pigments. In minerals that contain a mix of cations, the color will, naturally, be a mix of the cations' particular colors. | |||
=====Calcium===== | |||
Calcium is the most common cation in biomineral salts here on Earth - as found in the bones of vertebrates and the shells of many sea creatures. This is unsurprising, given that, while it is less biogically abundant than sodium and potassium, which don't form structural biominerals, it is much more abundant in biology than any other metal cation that ''does'', playing vital roles in an enormous variety of biochemical processes, from the nerve activations of metazoans to the opening and closing of stomata in plants. Even alien organisms that primarily use some other cation in their biominerals are likely to use calcium as a substantial component as well. | |||
Calcium salts do have a particular weakness, however, although it is not one that is ''typically'' relevant on Earth - they tend to be highly soluble in even relatively weakly acidic solutions. Many alien worlds may have waters too acidic for calcium salts to be viable as biominerals - indeed, Earth's own oceans were likely once too acidic for calcium carbonate to exist, and human pollution of the Earth's waters is making it more difficult for organisms to maintain calcium carbonate shells. Such cases offer opportunities to allow other metal cations to shine (as primary components of biomineral salts) - though, there is no particular reason biominerals primarily using other metals couldn't just appear anyways on occasion. | |||
Calcium minerals tend to be very opaque whites - as popular knowledge might already tell you. | |||
=====Magnesium===== | |||
Magnesium is about half as abundant in biochemical systems as calcium, here on Earth, and also plays a variety of incredibly important roles therein - including being a component of ATP/ADP, and the chlorophyll plants use for photosynthesis. | |||
Magnesium salts are substantially less soluble in weak acids than calcium salts, meaning they are potentially favored as biominerals over calcium salts on more acidic planets. They also tend to be slightly harder and stronger than calcium salts. | |||
Magnesium minerals tend towards light, greenish-grays. | |||
=====Manganese===== | |||
Manganese is yet another abundant cation in biochemical systems, though it is significantly rarer in Earth's crust than either calcium or magnesium (being about as common as phosphorous - which, given phosphorous is a common component of biominerals, is easily enough for structural usage). It is one of the most common metal cofactors found in enzymes, and thus plays an enormous role in biochemistry. | |||
Unlike calcium and magnesium, which are found diffused throughout the world's waters, manganese tends to be concentrated in particular locations - particularly, around hydrothermal vents, which carry manganese from deep in the Earth to the ocean, or near the mouths of rivers, which dissolve manganese from sediment as they run to the sea. In the open ocean, manganese content is essentially nil - meaning organisms that live there must obtain it from the food chain, from organisms that were able to obtain it in coastal waters or those around hydrothermal vents. | |||
Like magnesium salts, manganese salts tend to be less soluble in weak acids than calcium salts, and are also slightly harder and stronger. They also tend to be notably ''heavier and denser'' than either calcium or magnesium salts - rhodochrosite, for instance, has a specific gravity of about 3.7, compared to calcite's ~2.7. | |||
Manganese minerals tend strongly towards bright, vibrant pinks - even in minerals where manganese makes up less than half of the cations: some kutnohorite specimens that are only ~40% manganese, and nearly ~60% calcium, are still vibrant pink colors. | |||
=====Iron===== | |||
Iron is abundant in both biochemical systems and in Earth's crust (in the latter case, far more so than any other metal, save silicon and aluminum, which are biochemically inert). Yet, there is very little of it in Earth's oceans, and it is only occasionally used in biominerals. Why? | |||
The answer has less to do with the commonality of iron, or its solubility in water, than with how it reacts with oxygen. Prior to the Great Oxygenation Event, Earth's own oceans were replete with dissolved iron; it is believed it was far more commonly used in biochemistry than even today, as well, with organisms performing photosynthesis using iron and sulfur instead of carbon dioxide and water. However, as oxygen was added into Earth's atmosphere - and thus dissolved into the oceans as well - it began to react with the dissolved iron, forming iron oxides which precipated out, sunk to the seafloor, and became buried in sediment. | |||
This means the bioavailability of iron in a planets waters has less to do with the sheer amount of iron on the planet - and more to do with how long it is able to last before becoming oxidized in the planet's atmosphere. In heavily oxidizing atmospheres, iron's bioavailability will be drastically curtailed - and in atmospheres less oxidizing than Earth's, it will be increased. | |||
Iron minerals tend to range from black to orange-red. | |||
=====Copper===== | |||
Copper, while less abundant than iron, also plays enormous roles in biochemical systems throughout Earth's biosphere. It is especially important in electron and oxygen transport; all eukaryotes depend on copper-containing proteins for aerobic respiration, and the copper-containing hemocyanin is a common alternative to hemoglobin for carrying oxygen in blood among Earth life (and should be prevalent among alien life, too - especially on cold worlds with relatively low oxygen pressures, where it is more efficient than hemoglobin). | |||
Similarly to iron, dissolved copper in water will, in an oxidizing atmosphere, slowly convert to copper oxide, which tends to precipitate out of solution; its bioavailability, therefore, is again limited more by its half-life in the world's waters than by its raw abundance. It is, however, slightly more soluble than iron oxide - notably, it dissolves in aqueous ammonia, and, as has already been noted, many alien worlds can be expected to have oceans of aqueous ammonia. | |||
Copper biominerals are found in a number of clades on Earth, usually playing specialized roles similar to those played by iron; they are found in the jaws of bloodworms and the mycelia of certain fungi. | |||
Copper minerals tend to be very vivid, cool colors, ranging from blue-green to bright blue. | |||
=====Strontium===== | |||
The presence of strontium in this list may be surprising, given its high atomic number and relatively low level of biological activity, but strontium is actually found as a primary component of biominerals on Earth - particularly, in the [https://en.wikipedia.org/wiki/Acantharea Acantharea], which possess skeletons of strontium sulfate. It is surprisingly common as an ion in Earth's waters, owing to how easily it tends to be absorbed out of rock and soil, similarly to calcium. | |||
Overall, strontium minerals tend to have near-identical physical and chemical properties to their calcium equivalents - save for the fact that strontium minerals tend to be much, much heavier and denser. This is believed to be part of the reason why the Acantharea evince skeletons of strontium sulfate - the heavy weight of the strontium helps them sink to the ocean floor, their preferred habitat. | |||
Strontium minerals tend to be either white, like their calcium equivalents, or light, ethereal blues. | |||
=====Barium===== | |||
The presence of barium here is likely even more surprising than strontium, given it is even less biologically active - but, again, its biomineralization is demonstrated in Earth life, typically as an accessory component to strontium biominerals. Biomineralization of barium sulfate is notably found in the Desmidiales, a clade of unicellular algae closely related to modern plants. | |||
Not only is barium not particularly biochemically useful, its high reactivity renders it highly toxic to virtually all biological systems. One might think this is contrary to its use as a component of biominerals, but, in fact, barium occurs as a component of biominerals not despite, but ''because of'' its toxicity. In the form of mineral salts, barium's reactivity is neutralized; it is no longer a danger to the organism, once bound into salt crystals. As a result, many organisms have evolved the ability to secrete crystals of barium salts, in order to remove the toxin from the environment - and some then further evolved the ability to incorporate it into their structural materials. | |||
Barium minerals are generally white or colorless. | |||
====Anions==== | |||
Salts always involve a cation and an anion, and in the case of biominerals, the anion is always, specifically, an oxyanion - the common monatomic anions are too reactive, and the polyatomic anions which use nitrogen in the place of oxygen (such as cyanide and cyanate) are too unstable in oxidizing atmospheres (but may see more use in exotic biochemistries, such as in alkane solvent life). | |||
The physical properties of mineral salts are typically more dependent upon the anion than the cation, perhaps surprisingly - for instance, the vast majority of phosphate minerals are harder and stronger than the vast majority of carbonates. | |||
Unlike with the cations, mineral salts typically include only a single anion; this applies to both biogenic and inorganic minerals. | |||
=====Carbonate===== | |||
Carbonate (CO₃²⁻) is by far the most common anion found in biomineral salts in life on Earth - and is likely to be so on alien worlds as well. It is utterly ubiquitous throughout the world's waters (and thus, carbonate biominerals will be far more limited by the availability of their cations than by the availability of carbonate), and the same should apply to virtually any alien ocean - carbonate readily forms wherever carbon dioxide and water interact, and on a world with carbon dioxide in its atmosphere (which should be any alien world capable of sustaining life-as-we-know-it), that becomes "anywhere there is water". | |||
Carbonates tend to be slightly more soluble in weak acids than the other common biomineral salts (though it depends on the cation as well - magnesium carbonate is less soluble than calcium carbonate, for instance). | |||
<gallery mode=nolines heights=300px> | |||
File:Foraminifères de Ngapali.jpg|thumb|Calcite tests of foraminifers. | |||
File:NautilusCutawayLogarithmicSpiral.jpg|thumb|Cutaway of a nautilus shell, made of nacre - tiny aragonite plates layered on top of each other. | |||
</gallery> | |||
=====Oxalate===== | |||
Oxalate (C₂O₄²⁻), another carbon-containing anion (structurally, essentially two carbonates joined together by replacing the double-bonded oxygen with a bond between the two carbons), is also found in biomineral salts on Earth, though typically only in minor roles - though, it may easily be far more important in alien life! | |||
Oxalate, unlike carbonate, does not appear to be generated by inorganic processes; it is instead ubiquitously produced by plants (and a single genus of mold - [https://en.wikipedia.org/wiki/Aspergillus ''Aspergillus'']), through incomplete oxidation of carbohydrates. It is, in fact, in human health, most notable for the form of biomineralization that occurs when consuming too much of foods high in oxalate/oxalic acid - the formation of kidney stones, which largely consist of calcium oxalate, as the oxalate bonds to calcium ions in your gut and forms solid crystal precipitates. | |||
Oxalates tend to be somewhat harder and stronger than their carbonate equivalents, though not as much so as phosphates. | |||
=====Phosphate===== | |||
Phosphate (PO₄³⁻) is, perhaps surprisingly, not actually that common on earth, despite the fact that your very own bones are primarily composed of hydroxyapatite (Ca₁₀(PO₄)₆(OH)₂), and phosphate minerals of inorganic origin are quite common. Only vertebrates and the mantis shrimp (very specifically the mantis shrimp - no other arthropods) have been shown to have independently evolved phophate biomineralization; this leaves it having evolved only twice, compared to the dozens of times carbonate biomineralization has evolved. | |||
The reason why phosphate biomineralization is so (evolutionarily) rare is not particularly well understood; it may be that the more complex crystalline structures of phosphates (relative to carbonates) do not occur to biological systems so easily, or that phosphate (itself a very essential component for life) is too precious to waste on structural material for most species, or some other reason. Regardless - this implies it is far from likely that alien organisms will have bones (or other mineralized components) made out of the same stuff as our own! | |||
It is perhaps better to address the evolution of phosphate biomineralization not from the persepctive of ''why'' rather than ''why not?'' - it is, after all, far from the default, even if we tend to (in this case, erroneously) assume our own biochemistry's way of doing things is "normal". While there are multiple theories, one I've personally found convincing for how vertebrates evolved phosphate biomineralization is that it was deeply tied to the origins of vertebrate bone as a form of essential nutrient storage - not only is phosphate itself an essential nutrient, and storing it along with calcium a potentially useful adaptation in and of itself, primitive vertebrates would undergo periodic changes in the pH of their blood based on level of activity - changes in pH that might damage carbonate biomineral deposits, but which phosphate biominerals would be more resistant to.<ref name="VertebrateBone">[https://www.jstor.org/stable/2409087]</ref> | |||
Phosphate biominerals tend to be dramatically harder and stronger than carbonates, and slightly denser and heavier. | |||
=====Sulfate===== | |||
Sulfate (SO₄²⁻) is yet another common anion in biomineral salts; substantially more common than phosphate, but much less so than carbonate. | |||
Sulfate biominerals are, in fact, generally marginally softer and weaker than their carbonate equivalents, which one might imagine would make them worse as structural materials; one must wonder why organisms bother, when carbonate is so ubiquitous. The answer has to do with some of the particular chemical properties of sulfate. | |||
For one, sulfates tend to be hygroscopic without being hydrophilic - they tend to absorb water from the environment, but don't tend to dissolve easily. This already makes them more viable in environments where carbonates are not, due to the relatively high solubility of carbonates; but it also allows a slightly odd capacity to tweak the properties of sulfate minerals via controlling their level of hydration. While sulfate is, itself, heavier than carbonate, and sulfate minerals therefore tend to be heavier as well (again, as shown in the case of strontium sulfate), highly hydrated sulfates will actually be lighter and less dense than carbonates; this means organisms can effectively "tune" the density of their sulfate biominerals over the course of their evolution, via the level of absorbed water present in the crystal structure, evolving them to be lighter or heavier as the organism adapts to different environments. | |||
Second, sulfate tends to much more easily bind metal ions and precipitate out of solution than carbonate does; this makes sulfate biominerals generally cheaper, energetically, to construct, and makes them preferred for capturing relatively rare cations (such as the aforementioned strontium or barium). | |||
==Polyesters== | |||
Given that polyesters are typically thought of as synthetic polymers, it may be surprising to hear that polyesters do, in fact, occur and play structural roles in Earth life - although, in only two known cases, both of which are relatively marginal. In particular, the cutin that makes up the waxy outer layer covering the leaves of plants; and a special polymer secreted by bees of the genus ''Colletes'' to protect their brood cells. Both cases are, unfortunately, poorly understood - but they show, without a doubt, that polyesters are viable as a structural biological material, and there's no reason why alien organisms might not make much wider use of them! Some bacteria also make use of polyhydroxylalkanoates as an energy and carbon storage mechanism, but it seems to serve no structural purpose. | |||
Polyesters tend to be divided into two categories, based on the motifs present in their chemical structure, which are, incidentally, also the two classes of hydrocarbon: '''aliphatic''' polyesters, where carbon atoms form open chains; and '''aromatic''' polyesters, where the carbon atoms form closed, six-member rings. Aromatic polyesters tend to be much more crystalline in structure (and therefore much harder and more rigid), while also being more chemically and thermally stable. Most are, however, rather difficult for organisms to synthesize. | |||
While the most well-known synthetic polyester - and an obvious thought when hearing that polyesters are viable structural biological materials - is polyethylene terephthalate, otherwise known as PET, the conditions under which this is produced are not suitable for biological processes. There are, however, a number of other interesting polyesters to look at. | |||
===Polyesters from hydroxy acids=== | |||
All polyesters that appear in nature here on Earth - although, this is a rather limited selection, given their relative rarity - are, it seems, derived from hydroxy acids (that is, carboxylic acids with at least one hydroxyl group), particularly short-chain fatty acids. The exact chemical mechanisms by which biochemical processes produce these polyesters is generally poorly understood, although polyhydroxylalkanoates are known to be produced via action of an enzyme on the hydroxy acids. | |||
====Cutin==== | |||
[https://en.wikipedia.org/wiki/Cutin Cutin], along with the related polymer '''suberin''', is what provides plants with the tough, waxy outer coating of their leaves, assisting them in retaining their structure and making them hydrophobic so that rain slides off their surfaces. While it is not very well understood, it is thought to be a branched polymer of C16 and C18 hydroxy fatty acids, such as hydroxy palmitic acid. These very-long-chain acids, and its branching structure, result in an amorphous, soft, waxy structure, that is nonetheless fairly tough, completely insoluble in water, and highly hydrophobic. | |||
====Polyhydroxylalkanoates==== | |||
[https://en.wikipedia.org/wiki/Polyhydroxyalkanoates Polyhydroxylalkanoates], such as polyhydroxylbutyrate, are another polyester that appears in nature - albeit not for structural purposes. It is used by a number of species of bacteria for energy and carbon storage. Nevertheless, it still provides an option to us for a structural biopolymer, as, on Earth, polyhydroxylalkanoates are routinely harvested from the bacteria that produce them and used to make strong plastics, particularly for biomedical use (as they are non-toxic). | |||
As a structural material, polyhydroxylalkanoates are generally quite ductile, and may or may not be quite elastic depending on exact composition. They are tough and strong, with similar tensile strength to polypropylene. They are generally permeable to oxygen, but ''not'' to water. Compared to other polyester plastics, they also tend to have quite good UV resistance. | |||
====Phenolic acid polyesters==== | |||
[https://en.wikipedia.org/wiki/Phenolic_acid Phenolic acids] - that is, a phenol with a carboxyl group, such as salicylic acid<ref name=Polysalicylate>https://pubs.acs.org/doi/10.1021/acsmacrolett.9b00890</ref> - which are widely found in nature, have been demonstrated to be capable of forming polyesters under a variety of conditions. In the case that these are homogenous, linear polyesters (i.e. have no chain branches), their properties should generally be similar to other aromatic polyesters - that is, highly crystalline, and therefore hard and rigid. | |||
More complex polyphenolic acids may instead more closely resemble polyphenols - indeed, phenolic-acid polyesters are both polyphenols and polyesters! | |||
===Polyesters from lactones=== | |||
[https://en.wikipedia.org/wiki/Lactone Lactones] can be readily converted into polyesters via a variety of catalytic ring-opening polymerization reactions. While there are, as far as I am aware, no natural polyesters that are derived this way, lactones do occur in nature, and catalytic processes are well-suited for biochemistry. | |||
====Polylactic acid==== | |||
[https://en.wikipedia.org/wiki/Polylactic_acid Polylactic acid], most often known by its acronym "PLA", is a plastic most known, here on Earth, for its use as a 3D-printing filament. While it is not found in nature, it is catalytically synthesized from lactide (the lactone of lactic acid), which very much is, meaning alien organisms that synthesize it are highly plausible. | |||
Unlike most other aliphatic polyesters, PLA ranges from semi-crystalline to highly crystalline in structure depending on exact composition, and is thus quite hard and rigid - it is somewhere in between polystyrene and PET in mechanical properties. | |||
PLA is also sensitive to UV degradation, but this simply breaks off monomers, which could be biologically recuperated and the PLA repaired. | |||
====Polycaprolactone==== | |||
WIP - needs more research | |||
====Polybutyrolactone==== | |||
WIP - needs more research | |||
=Credit= | |||
Author: DocViviLeandra | |||
=Footnotes:= | |||
[[Category:Life]][[Category:Biochemistry]][[Category:Chemistry & Materials]] | |||
Latest revision as of 13:54, 23 April 2024
Real organisms use a wide variety of structural materials to give toughness, strength, rigidity, and other desirable material properties to their bodies, both on the cellular (as in cell walls) and tissue (as in bones or exoskeletons) level. Moreover, these materials are rarely homogenous - biological structural materials are almost always composites of more than one material, becoming something greater than the sum of its parts.
Rather than simply take the approach of listing options, this page will aim to explore the general categories of structural biological materials - both real and theoretical - and explain how their particular properties play into how they're used in living creatures.
Polysaccharides
Polysaccharides are perhaps the most widespread - and simplest to evolve - structural materials we find in living creatures. Saccharides, especially glucose, are universally used as energy storage in Earth life, and it's likely that alien life - at least, that which shares our basic biochemistry - will do the same. It therefore isn't much of a step to derive your structural materials from the stuff you already use for energy storage!
Even though monosaccharides are typically hydrophilic, and readily dissolve in water, most polysaccharides are, in fact, quite hydrophobic and insoluble. This makes them quite well-suited for assembly-on-the-spot - the component monosaccharides can be dissolved in the cellular medium for transport, and will precipate out of the water as they are assembled into polysaccharides.
One defining element of polysaccharides, on the chemical level, is the multiple free hydroxyl groups on each monomer of the chain. Through a variety of processes, these can be replaced by other functional groups, altering the properties of the polysaccharide; they may also serve as useful bonding sites for other structural molecules, such as peptides and polyphenols. This chemical versatility is another reason why they are so ubuiquitous in Earth life - they excel at a wide variety of structural purposes, and make a very good "foundation" for other structural biological materials to build on in composite materials.
Cellulose, and other simple linear polysaccharides
Cellulose is the simplest and most widespread polysaccharide that sees structural use on Earth, and is likely to be so on alien worlds as well. It's a simple linear chain of β(1→4) linked units of d-glucose - the same molecule nearly all life on Earth uses to store energy. Aliens might use ʟ-glucose instead, but this should have identical mechanical properties.
Contrary to popular belief, cellulose is actually quite flexible, when alone. The rigidity of plant cells - famously with cell walls made out of cellulose - is provided by other materials bonded with cellulose, such as lignin, along with the hydrostatic pressure provided by the water within the cell.
A number of Earth organisms are demonstrated to use mannose, or other simple sugars, in the place of glucose, forming structural polysaccharides such as mannan. Their properties are similar to cellulose - though, how they may bond to other polymers might differ, as we'll discuss later with glucomannans.
Some other organisms, such as oomycetes, use other β-glucans - that is, linear chains of glucose - which have different linkages (such as the β(1→3) linkages of chrysolaminarin) as structural materials. In bulk, their mechanical properties are fairly similar.
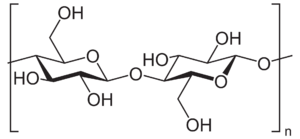
Hemicelluloses - heterogenous simple polysaccharides
In plants, cellulose is not found alone - it is typically accompanied by filaments of hemicellulose, otherwise known as polyose, a group of highly heterogenous polysaccharides composed of a semi-random mix of miscellaneous saccharide molecules - predominately arabinose and xylose. The heterogeneity of these fibers inhibits the formation of crystalline structures the way cellulose might form, rendering the result relatively amorphous and weak: if isolated, hemicelluloses would have a gum- or gel-like texture. While that may seem undesirable in a structural material, hemicelluloses perform important functions in plants - in particular, cross-linking cellulose fibers together, adding additional strength and toughness to the resulting matrix.
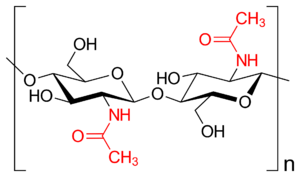
Chitin, and other poly-amino-sugars
Chitin is the second most widespread structural polysaccharide on Earth, and for good reason. Like cellulose, it is a linear chain of single monomers. Rather than being made of simple glucose, however, it is composed of N-acetylglucosamine - an amino-sugar of glucose where one of the hydroxyl groups is replaced by an acetylamino moiety. This acetylamino moiety forms stronger hydrogen bonds with adjacent polymers than the hydroxyl group would, rendering chitin much stronger and slightly more rigid than cellulose - though, it still remains plenty flexible, without other components to rigidify it.
N-acetylmannosamine and N-acetylgalactosamine, derivatives of mannose and galactose, respectively, also exist in nature - to my knowledge, they are not used to form polymers like chitin, but an alien organism may do so!
Hydrophilic polysaccharides
Though most polysaccharides are hydrophobic, this does not apply to all. Many are possessed of an overall negative charge and/or are internally polar, allowing them to become quite hydrophilic. As hydrophilic molecules, they will readily dissolve in water - a characteristic exploited in certain biological lubricants, such as the fluid that fills the space between joints in vertebrates, which consists of relatively short chains of hydrophilic polysaccharides dissolved in water (when you pop your knuckles - or other joints - what you're doing is popping bubbles in this fluid).
However, though these polysaccharides dissolve in water, this does not break the bonds of the polymers. This is a property of water-soluble polymers in general - though they may "dissolve", they do not break apart. This means relatively long polymers, especially those with extensive cross-linkages between them, will maintain their structural form when hydrated - instead, the water infiltrates the polymer structure, causing it to swell into a sort of hydrogel or gum, which may be stiffer or softer depending on how closely interlinked the polymer chains within it are. Water can also pass through the polysaccharide gel, at a rate which is, again, dependent on the exact structure of the gel - which may assist organisms, particularly unicellular or colonial ones, in osmoregulation. This structural usage of hydrophilic polysaccharides is obviously better suited for aquatic biota, which do not have to worry about their polysaccharide hydrogels drying out - but can still be quite useful in the interior structures of terrestrial biota.
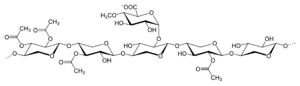
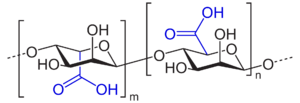
Algin, and other polyuronic acids
Algin, or alginic acid, a polyuronic acid, is a primary structural component of the cell walls of brown algae, such as kelp. It is a polymer of two uronic acids in alternating sequence - β-D-mannuronate and α-L-guluronate. Uronic acids are derivatives of saccharides where the hydroxyl group opposite the carbonyl has been oxidized to a carboxyl group, making them a kind of carboxylic acid. This makes polyuronic acids acidic (as implied by the name) - which in turn makes them hydrophilic and water-soluble.
The hydrophilic nature of algin is responsible for the gummy, gel-like texture/structure of the brown algae.
The carboxyl group replacement that defines the uronic acids very rarely co-occurs with replacements of other hydroxyls on the same monomer with other functional groups, for reasons I don't personally understand. For example, I know of only one amino uronic acid - N-Acetyltalosaminuronic acid.
Sulfated polysaccharides
Sulfated polysaccharides are common throughout Earth life, and should be expected to be common among alien organisms with broadly similar biochemistry. In sulfated polysaccharides, one or more free hydroxyl groups are replaced by a sulfate ion; in some cases, sulfates occur only once every several monomers, while in others, nearly every single hydroxyl group in the entire chain is replaced by a sulfate.
Like polyuronic acids, sulfated polysaccharides are hydrophilic, due to the negative charge of the sulfate ion. However, they have a range of properties which distinguishes them profoundly from their uronic acid equivalents.
First, whereas the carboxyl groups that define the uronic acids rarely co-occur with other hydroxyl-replacements on the same monomer, sulfation frequently occurs with other functional group replacements - particularly, sulfated amino sugars, where one or more of the remaining hydroxyl groups (after the addition of the amine) has been sulfated, are quite common in nature, allowing a single monomer to bear the characteristics brought by both sulfation and other derivative processes. This extends to, as previously mentioned, molecules where each monomer has had all free hydroxyl groups sulfated, resulting in a much greater negative charge (and thus, greater hydrophilicity) than in uronic acids.
Second, sulfated polysaccharides are quite good at binding and precipitating metal ions, such as calcium or sodium, out of solution. Relatively short sulfated polysaccharides, therefore, often play a non-structural role in regulating the transport of ions throughout an organism; and sulfated polysaccharides in the outer surfaces of aquatic organisms, such as in the cell walls of many algae, may assist in the capture of necessary ions from the water. However, this also lends itself to a structural purpose - sulfated polysaccharides appear to play a role in biomineralization in some clades, such as in the coralline algaes, precipitating metal ions out of solution to form hard, mineralized coatings on the organism's exterior.
Disaccharide polysaccharides
As demonstrated by algin, above, many structural polysaccharides, rather than consisting of a homogenous chain of a single monosaccharide molecule, are instead often composed of two distinct monosaccharides in alternating sequence. This allows the copolymer to have some of the properties offered by both monosaccharides.
Glycosaminoglycans
Glycosaminoglycans, also known as mucopolysaccharides, are a common form of alternating polysaccharide, consisting of alternating units of a uronic acid and an amino sugar, the latter of which may be sulfated (as in heparin) or not (as in hyaluronan). These polysaccharides are very polar, and attract water very strongly as a result, while also self-adhering quite well.
Glycosaminoglycans fulfill a variety of important functions, particularly in animals. Since diffusion through them is possible, but quite slowly, they help slow and regulate the intercellular diffusion of substances like ions and proteins; since they can adhere quite well, they serve as an adhesive binding different cells together; and since they attract water so well, they are used to assist in the retention of moisture where watery surfaces must be exposed, such as in the eyes of vertebrates.
Peptidoglycan
Peptidoglycan, also known as murein, is the primary structural component of bacterial cell walls. It is an alternating copolymer of N-acetylglucosamine (as found in chitin) and N-acetylmuramic acid - a complex monosaccharide where a lactic acid moiety has replaced yet another hydroxyl group on N-acetylglucosamine. Like in chitin, the acetylamino moieties serve to assist in the formation of crystalline structures through strong hydrogen bonds; the addition of the lactic acid to every other monomer in the chain, however, serves as a strong binding site for short oligopeptides (consisting of three to five amino acids) which bind to a different polysaccharide chain at each end, forming a regular mesh that resembles a chain-link fence.
The resulting structure is very hard to break apart chemically, and strong enough to maintain the regular shape of cells observed in most bacteria. There is no particular reason, in fact, why it is not found in eukaryotes or archaeans, other than these two groups simply not having evolutionarily stumbled upon it - peptidoglycan only evolved once. So, complex alien life might use it!
Branching polysaccharides
As seen above, almost all polysaccharides used for structural purposes in living organisms are linear chains, though branching polysaccharides are commonly used for energy storage. However, some branching polysaccharides do, in fact, see structural use - such as the glucomannans found in some yeasts and plants. Glucomannans consist of a linear chain of mannose units (called a mannan), with short glucose branches. Unlike linear polysaccharides, branched polysaccharides resist forming neat, crystalline structures (as the branches interfere with hydrogen-bond formation between polymers), and thus are able to slide past each other, forming a rubbery, hydrophilic solid, or even a viscous liquid (when hydrated).
Polyphenols (lignin, tannin, etc.)
Polyphenols, such as lignin and tannin, are a broad category of biopolymer found almost exclusively in plants on Earth, consisting of many phenol units oxidatively coupled together. Unlike other common structural biopolymers, polyphenols are typically non-linear - instead forming complex 3-dimensional structures. They are also often highly heterogenous, made of many different monomers. This makes it difficult to talk about sub-types of polyphenol, as we have done for polysaccharides - instead, this section will talk about the properties different polyphenols often possess.
Color
Polyphenols' complex 3d structures leads to a variety of interactions with light waves that result in them being typically opaque and strongly colored. While most polyphenols are dark brown - lignin being responsible for the dark brown color of most tree bark - some polyphenols have brilliant colors, such as the anthocyanidins that provide flower petals with vivid reds, purples, and blues.
While structural polyphenols are typically quite separate from pigment polyphenols, an organism that is able to synthesize one is likely capable of synthesizing the other!
Reactivity
Whereas most structural biopolymers are only weakly reactive, polyphenols are quite reactive, specifically to oxidation. This might sound like a vulnerability for a structural material, but polyphenols' reactivity is usually localized to a few specific sites on the molecule. This makes them very good at binding to other structural polymers, like polysaccharides and proteins, at multiple sites, via oxidative coupling. Other structural biopolymers bound by polyphenols in this way tend to become exceptionally hard and rigid - like tree bark.
Resistance to degradation
Some - not all - polyphenols, such as lignins, are exceptionally resistant to bio-degradation, making them very difficult for other living things to break down and digest - and this extends to the other polymers they bind to. This makes them very good at protecting from penetration by parasites or pathogens, and limits the possible predators of organisms that use polyphenol cross-linking in their structures.
Affinity for saccharides
Polyphenols have something of a special affinity for saccharides, and will not only readily bond with them, but also often include one or more saccharide molecules in their structure - such as in tannic acid, a polyphenol composed of phenol branches stemming from a glucose molecule at its center.
Cross-linking proteins (collagens, keratins, sclerotins, etc.)
Proteins - perhaps unsurprisingly, given their ubiquity - are common components of structural biological materials, specifically in the form of cross-linking proteins like keratins or sclerotins, which bond to each other at many different sites, allowing for not only toughness and rigidity but a tunable degree of both - via controlling the frequency of cross-linkages, materials can smoothly vary in rigidity and hardness, often forming distinct gradients in biological structures (such as arthropod cuticle becoming more flexible around the joints).
Most cross-linking structural proteins tend to be brown to black in color, though are not particularly opaque, and some can be outright colorless. Their versatility also lends themselves to the creation of complex microstructures resulting in vivid structural colorations, and tissue that includes them often also plays host to a variety of pigments that add their own colors to the material.
Collagens
Collagens are the primary family of structural proteins found in all metazoans, though they are most prominent in the deuterostomes - in protostomes, many of the functions typically performed by collagens are instead performed by chitin. In mammals, collagens make up 25-35% of all proteins in the body! They make up the connective tissue, while also adding strength to other tissues (such as blood vessels) throughout the body, and also providing the matrix on which hydroxyapatite is deposited to form bone and cartilage in vertebrates.
Collagen consists of a left-handed triple helix of elongated peptide chains. About half of its amino acid content is glycine and proline alone, which appear to play a role in binding the three components of the helix together. In addition, sites throughout the chain are saturated in hydroxylysine - a hydroxylated derivative of the amino acid lysine. It is by these hydroxylysine sites that collagen helixes are cross-linked - via aldol condensation, strong covalent bonds are formed through conjugated enone moieties between the hydroxylysine sites on adjacent collagen helixes. This binds the adjacent helixes together very strongly, while still allowing hydrolysis of the linkages when they must be split apart, unlike certain other structural proteins.
Unlike most structural proteins, collagen appears colorless, or sometimes white. It is also extremely flexible - collagenous tissue must rely on other components to add hardness and rigidity, if necessary.
Keratins
Keratins are the primary family of structural proteins found in vertebrate integument - composing hair, nails, feathers, scales, etc, while also reinforcing the epithelial tissue (the outermost layer of the organs and blood vessels). There are two primary categories of keratin - α-keratins, which are found in all vertebrates, and β-keratins, which are found only in sauropsids. The primary difference between these is that α-keratins form double-helix filaments and are more flexible, whereas β-keratins form β-pleated sheets and are significantly harder and more rigid.
Both types of keratin crosslink by means of disulfide bridges - they contain large amounts of the amino-acid cysteine, which bonds to cysteines on adjacent keratin polymers via the sulfur. These bonds are very strong and rigid, and, as mentioned before, the precise degree of hardness and rigidity can be precisely controlled by determining the frequency of disulfide bridges. The presence of so much sulfur-containing cysteine is why heavily-keratinized tissues - like human hair - smell pungent when burned.
Sclerotins
Sclerotins are another widespread family of structural proteins, found primarily in arthropods but also some annelids. Sclerotins are a much broader family of proteins than keratins, and do not have a particular identifiable geometric structure, unlike the α-helixes and β-pleated sheets of keratins. They are also not bonded by disulfide bridges - instead, they are cross-linked by a process, called sclerotization, similar to what is done to tan animal hide into leather.
Sclerotin proteins contain large numbers of free amine and thiol groups, which tend towards oxidative reactions with other molecules. In the process of sclerotization, quinones are enzymatically introduced to tissue containing large numbers of sclerotins, typically by enzymatic action on dopamine-derivatives such as N-acetyldopamine. These quinones then react, forming strong covalent bonds, with the amine and thiol groups on the sclerotins, cross-linking them together into an exceptionally tough, hard, and rigid material. Like keratins, the exact degree of hardness and rigidity can be controlled, this time via the amount of quinones introduced into the tissue.
Sclerotization is effectively non-reversible - cross-linked sclerotins being extremely hard to break apart - and thus organisms must shed sclerotinized parts if they need to change them, hence the necessity of molting in arthropods.
Some annelids, such as the intimidatingly-named bloodworm, have special sclerotins which bind metal ions, such as copper and iron, prior to undergoing sclerotization. This results in unique metal-composite structures which are exceptionally hard and strong.[1]
Other cross-linking proteins
Other cross-linking proteins are found in other clades, though none as archetypical and widespread as keratins and sclerotins.
For example, cephalopod mollusks possess their own particular cross-linking proteins that add hardness and rigidity to their beaks, operating in a two-tiered system - they are, unfortunately, absent a catchy name like keratins and sclerotins, and are instead simply referred to as chitin-binding beak proteins (CBPs) and histidine-rich beak proteins (HBPs). CBPs simply bind to multiple chitin chains at both ends, forming cross-links between them. HBPs add a second tier to this matrix, and bond to both CBPs and each other, forming an additional set of cross-links to add even more strength, hardness, and rigidity to the result.[2]
Alien organisms may be expected to exhibit yet more variations on the common motif of proteins cross-linked together, unseen on Earth - instead of directly using one found on Earth, it may be better to simply specify a, say, "keratin-like protein"!
Biominerals
While all the structural materials discussed previously - and those which will be discussed after - are polymers, mineral crystals also play a major role in structural biological materials. Biominerals excel at adding hardness, strength, and rigidity to biological structures; only a handful of organic polymers are capable of matching even the softest biominerals in hardness and rigidity.
For obvious reasons, tissue that is heavily biomineralized cannot be flexible - although, semi-flexible partially-biomineralized tissue, such as cartilage in vertebrate, does exist in nature.
In addition to performing structural functions, biomineralized tissue also often functions as a reservoir of biologically-essential chemicals, such as metal ions, phosphate, and sulfate. In fact, many biomineralized structures in modern Earth organisms are believed to have evolved from what were initially purely storage deposits - for example, the bones of vertebrates are believed to be derived from what was initially the mere deposition of calcium and phosphate for storage.
All biomineralization processes found in nature (at least, that I am aware of) involve the creation of insoluble crystals from soluble components. The necessity of this particular combination of characteristics - soluble components, insoluble products - is obvious when you consider the challenges involved in creating them: chemicals must be soluble in water (or whatever alternative solvent) to be acquired from the environment and transported through an organism's tissues; and upon forming their insoluble products, they immediately precipitate out of solution, forming hard, insoluble crystals, and thus useful structural materials. This means that components that are insoluble cannot be acquired and transported, and products that are soluble cannot be made into useful structural materials. This renders certain sci-fi go-tos - such as giving an alien organism bones of titanium - implausible (as titanium is insoluble in water in all forms). However, there is still plenty of room to explore hypothetical biominerals, as will soon be demonstrated.
While most biominerals are salts - specifically, salts of metal cations and oxyanions - we will first discuss the exceptions to that rule.
Silicates

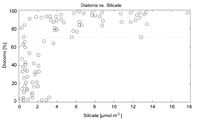
Silicates, otherwise known as common glasses (literally, like the kind in your window), are perhaps the most common and widespread kind of structural biominerals on Earth, having evolved independently countless times throughout Earth's tree of life. This may seem surprising, given that you probably know of very few living things that are made out of glass - and that's because almost all organisms that use silica as a structural biomineral are unicellular. While clades that use silica as a minor structural component are common among multicellular life here on Earth - such as the horsetail ferns - there is only one that is known to use it as the primary mineral component of their structural materials: the Hexactinellid sponges, otherwise known as the glass sponges.
Silicates are also one of the few biominerals thare are not typically found in the form of a salt - instead being typically formed of silica (SiO₂) in its various crystalline forms. Silica is extremely chemically inert, and therefore virtually useless to any living organism for biochemical processes - therefore, unlike many other biominerals, it is solely a structural material, and not a reservoir of essential nutrients. On the other hand, this non-reactivity makes it exceptional for structural purposes, and it is one of the hardest and strongest biominerals found in nature!
One may find the apparent ubiquity of biogenic silica confusing, in context of previous remarks on the necessity that biominerals be the insoluble products of soluble components, as silicates are not usually thought to be soluble - the truth, however, is that silicates are very weakly soluble, depending on their crystalline structure. While, ordinarily, such weak solubility would be insufficient to supply enough of the compound to a planet's oceans, the key lies in that silica makes up the vast majority of the Earth's crust, and can be expected to do the same on alien worlds of similar composition to Earth; thus, the relatively small portion of silica that dissolves is actually quite gigantic, in absolute terms! And once this dissolved silica has been re-formed into crystals, it does not readily dissolve again - thus fulfilling that same criterion.
Silicate biomineralization is typically performed by precipitating silicic acid - the dissolved form of silica - out of water, onto a matrix of specialized biopolymer filaments. This not only results in a very strong structural material, it is also, energetically, exceptionally cheap for the organism in question - organisms that use silica as a structural material spend only a fraction of the energy forming silicate structures as other biominerals require.
This, however, does mean that an organism's ability to build biological structures out of silica is strictly limited by the availability of silicic acid in its environment - and given how silicic acid enters the environment in the first place, this quantity is quite limiting indeed. In fact, competition over silicic acid in the biosphere of Earth is quite fierce: Hexactinellid sponges once were one of the dominant clades of filter-feeder throughout the water column, forming reefs comparable to today's corals, but are today limited entirely to deep water environments - by the Cenozoic period, the diatoms had become ecologically ascendant (though they are believed to have first appeared in the Triassic, they did not become ecologically dominant until much later), and nearly monopolized the supply of silicic acid in the ocean's photic zone, making it impossible for the shallow-water glass sponges to obtain enough to build their spicules. The implications of this for alien ecosystems are profound - in order for silica to be more widely-used in an alien ecosystem than it is on Earth, something will have to dramatically increase the availability of silicic acid in the planet's waters. As an example - adding ammonia or methanol to water substantially increases the solubility of silica, but not enough to make silica less useful as a biomineral; thus, the subsurface oceans of many ice worlds (provided the lower surface is silica, and not another layer of ice), thought to consist of eutectic mixtures of water and ammonia, may be replete with silica-biomineralizing life!
As a biomineral, silica is essentially always found in the form of opal, the hydrated, amorphous form of silica. This is, perhaps unfortunately, not typically what we would identify as precious opal - the gemstone known for its vibrant plays of color - but common opal, weakly translucent and milky-gray in color. Given that precious opal is merely a particular arrangement of hydrated silica, however, it is entirely plausible that an alien organism might evolve surface structures of precious opal as a form of display!
Metal Oxides and Sulfides
A few organisms, in need of exceptionally hard and tough biominerals, have evolved the use of transition metal oxides and sulfides - particularly the oxides and sulfides of iron. Unlike metal salts, they do not make particularly good reservoirs of essential metals for other biological purposes - they are quite strongly bound, and thus, like silicates, are almost solely used as structural materials. However, they do also, potentially, have one other interesting application - many of these minerals (particularly the iron-oxide magnetite and the iron-sulfide greigite) are ferromagnetic, and thus many organisms use them as components of magnetosomes - organelles for sensing geomagnetic fields, and a kind of biological compass.
It is not particularly common for these biominerals to occur as major structural components throughout an organism's body - rather, most cases involve, quite specifically, teeth. Metal oxides and sulfides are generally energetically and nutritionally expensive to form; therefore, they tend to be used for specialized purposes, with more common biominerals being used elsewhere. As an example, chitons and limpets both have independently-evolved iron oxide teeth (magnetite in the former case; goethite in the latter), which they use to scrape algae and other organisms from hard rocks on the seabed (their teeth being so exceptional at this that they are significant contributors to erosion) - however, both possess defensive shells of the rather more ordinary calcium carbonate, instead.
The exception, of course, occurs where metals to oxidize - and oxygen or sulfur to react them with - are easily found, such as around hydrothermal vents, which spew large amounts of sulfur and iron into the surrounding ocean. It is here that the snail Chrysomalion squamiferum, the scaly-foot snail, is found - with its shell and protective scales made of iron sulfides.
These oxides and sulfides of metals are typically formed by reacting oxygen or sulfur with metal ions inside of tissues, which then preciptates out as the corresponding metal oxide/sulfide.
Salts
As previously mentioned, the most common biominerals on Earth are mineral salts - particularly, salts of a transition or alkaline metal cation (such as calcium, magnesium, or iron) with an oxyanion (such as carbonate, phosphate, or sulfate).
As with other biominerals, the anion and the cation must be soluble in water; however, the resulting salt crystal must be insoluble, and thus able to precipitate out of solution. Unlike with silicates, which are always very weakly soluble, and with metal oxides and sulfides, which go from very soluble to completely insoluble, the gap between the solubilities of salts and their component ions is typically rather small; changing the pH of the solution may render insoluble salts soluble, or soluble salts insoluble, changing which make for workable biominerals.
Cations
While there are quite a large number of metals, both alkaline and transitional, that can potentially form salts with oxyanions, only a handful make for plausible components of biominerals. Most are simply too rare to be used in bulk for structural materials in biology. Many of those that remain are biochemically inert - particularly those that are completely insoluble in water, such as aluminum and titanium (sorry, SpecEvo writers who like giving their creatures titanium bones - it doesn't work! Titanium is biochemically inert). And some that are both common and very biochemically active, such as sodium and potassium, only seem to form salts that are soluble.
While we typically characterize biominerals as being composed of one cation in particular (e.g. we speak of our bones as being made from calcium), in reality, virtually all biomineral salts actually contain a mix of cations, though one might predominate - the bones of vertebrates actually contain significant amounts of magnesium and manganese in addition to calcium, for instance.
Notably, it is typically the cations, not the anions, that typically determines the color of mineral salts - in the absence of other pigments. In minerals that contain a mix of cations, the color will, naturally, be a mix of the cations' particular colors.
Calcium
Calcium is the most common cation in biomineral salts here on Earth - as found in the bones of vertebrates and the shells of many sea creatures. This is unsurprising, given that, while it is less biogically abundant than sodium and potassium, which don't form structural biominerals, it is much more abundant in biology than any other metal cation that does, playing vital roles in an enormous variety of biochemical processes, from the nerve activations of metazoans to the opening and closing of stomata in plants. Even alien organisms that primarily use some other cation in their biominerals are likely to use calcium as a substantial component as well.
Calcium salts do have a particular weakness, however, although it is not one that is typically relevant on Earth - they tend to be highly soluble in even relatively weakly acidic solutions. Many alien worlds may have waters too acidic for calcium salts to be viable as biominerals - indeed, Earth's own oceans were likely once too acidic for calcium carbonate to exist, and human pollution of the Earth's waters is making it more difficult for organisms to maintain calcium carbonate shells. Such cases offer opportunities to allow other metal cations to shine (as primary components of biomineral salts) - though, there is no particular reason biominerals primarily using other metals couldn't just appear anyways on occasion.
Calcium minerals tend to be very opaque whites - as popular knowledge might already tell you.
Magnesium
Magnesium is about half as abundant in biochemical systems as calcium, here on Earth, and also plays a variety of incredibly important roles therein - including being a component of ATP/ADP, and the chlorophyll plants use for photosynthesis.
Magnesium salts are substantially less soluble in weak acids than calcium salts, meaning they are potentially favored as biominerals over calcium salts on more acidic planets. They also tend to be slightly harder and stronger than calcium salts.
Magnesium minerals tend towards light, greenish-grays.
Manganese
Manganese is yet another abundant cation in biochemical systems, though it is significantly rarer in Earth's crust than either calcium or magnesium (being about as common as phosphorous - which, given phosphorous is a common component of biominerals, is easily enough for structural usage). It is one of the most common metal cofactors found in enzymes, and thus plays an enormous role in biochemistry.
Unlike calcium and magnesium, which are found diffused throughout the world's waters, manganese tends to be concentrated in particular locations - particularly, around hydrothermal vents, which carry manganese from deep in the Earth to the ocean, or near the mouths of rivers, which dissolve manganese from sediment as they run to the sea. In the open ocean, manganese content is essentially nil - meaning organisms that live there must obtain it from the food chain, from organisms that were able to obtain it in coastal waters or those around hydrothermal vents.
Like magnesium salts, manganese salts tend to be less soluble in weak acids than calcium salts, and are also slightly harder and stronger. They also tend to be notably heavier and denser than either calcium or magnesium salts - rhodochrosite, for instance, has a specific gravity of about 3.7, compared to calcite's ~2.7.
Manganese minerals tend strongly towards bright, vibrant pinks - even in minerals where manganese makes up less than half of the cations: some kutnohorite specimens that are only ~40% manganese, and nearly ~60% calcium, are still vibrant pink colors.
Iron
Iron is abundant in both biochemical systems and in Earth's crust (in the latter case, far more so than any other metal, save silicon and aluminum, which are biochemically inert). Yet, there is very little of it in Earth's oceans, and it is only occasionally used in biominerals. Why?
The answer has less to do with the commonality of iron, or its solubility in water, than with how it reacts with oxygen. Prior to the Great Oxygenation Event, Earth's own oceans were replete with dissolved iron; it is believed it was far more commonly used in biochemistry than even today, as well, with organisms performing photosynthesis using iron and sulfur instead of carbon dioxide and water. However, as oxygen was added into Earth's atmosphere - and thus dissolved into the oceans as well - it began to react with the dissolved iron, forming iron oxides which precipated out, sunk to the seafloor, and became buried in sediment.
This means the bioavailability of iron in a planets waters has less to do with the sheer amount of iron on the planet - and more to do with how long it is able to last before becoming oxidized in the planet's atmosphere. In heavily oxidizing atmospheres, iron's bioavailability will be drastically curtailed - and in atmospheres less oxidizing than Earth's, it will be increased.
Iron minerals tend to range from black to orange-red.
Copper
Copper, while less abundant than iron, also plays enormous roles in biochemical systems throughout Earth's biosphere. It is especially important in electron and oxygen transport; all eukaryotes depend on copper-containing proteins for aerobic respiration, and the copper-containing hemocyanin is a common alternative to hemoglobin for carrying oxygen in blood among Earth life (and should be prevalent among alien life, too - especially on cold worlds with relatively low oxygen pressures, where it is more efficient than hemoglobin).
Similarly to iron, dissolved copper in water will, in an oxidizing atmosphere, slowly convert to copper oxide, which tends to precipitate out of solution; its bioavailability, therefore, is again limited more by its half-life in the world's waters than by its raw abundance. It is, however, slightly more soluble than iron oxide - notably, it dissolves in aqueous ammonia, and, as has already been noted, many alien worlds can be expected to have oceans of aqueous ammonia.
Copper biominerals are found in a number of clades on Earth, usually playing specialized roles similar to those played by iron; they are found in the jaws of bloodworms and the mycelia of certain fungi.
Copper minerals tend to be very vivid, cool colors, ranging from blue-green to bright blue.
Strontium
The presence of strontium in this list may be surprising, given its high atomic number and relatively low level of biological activity, but strontium is actually found as a primary component of biominerals on Earth - particularly, in the Acantharea, which possess skeletons of strontium sulfate. It is surprisingly common as an ion in Earth's waters, owing to how easily it tends to be absorbed out of rock and soil, similarly to calcium.
Overall, strontium minerals tend to have near-identical physical and chemical properties to their calcium equivalents - save for the fact that strontium minerals tend to be much, much heavier and denser. This is believed to be part of the reason why the Acantharea evince skeletons of strontium sulfate - the heavy weight of the strontium helps them sink to the ocean floor, their preferred habitat.
Strontium minerals tend to be either white, like their calcium equivalents, or light, ethereal blues.
Barium
The presence of barium here is likely even more surprising than strontium, given it is even less biologically active - but, again, its biomineralization is demonstrated in Earth life, typically as an accessory component to strontium biominerals. Biomineralization of barium sulfate is notably found in the Desmidiales, a clade of unicellular algae closely related to modern plants.
Not only is barium not particularly biochemically useful, its high reactivity renders it highly toxic to virtually all biological systems. One might think this is contrary to its use as a component of biominerals, but, in fact, barium occurs as a component of biominerals not despite, but because of its toxicity. In the form of mineral salts, barium's reactivity is neutralized; it is no longer a danger to the organism, once bound into salt crystals. As a result, many organisms have evolved the ability to secrete crystals of barium salts, in order to remove the toxin from the environment - and some then further evolved the ability to incorporate it into their structural materials.
Barium minerals are generally white or colorless.
Anions
Salts always involve a cation and an anion, and in the case of biominerals, the anion is always, specifically, an oxyanion - the common monatomic anions are too reactive, and the polyatomic anions which use nitrogen in the place of oxygen (such as cyanide and cyanate) are too unstable in oxidizing atmospheres (but may see more use in exotic biochemistries, such as in alkane solvent life).
The physical properties of mineral salts are typically more dependent upon the anion than the cation, perhaps surprisingly - for instance, the vast majority of phosphate minerals are harder and stronger than the vast majority of carbonates.
Unlike with the cations, mineral salts typically include only a single anion; this applies to both biogenic and inorganic minerals.
Carbonate
Carbonate (CO₃²⁻) is by far the most common anion found in biomineral salts in life on Earth - and is likely to be so on alien worlds as well. It is utterly ubiquitous throughout the world's waters (and thus, carbonate biominerals will be far more limited by the availability of their cations than by the availability of carbonate), and the same should apply to virtually any alien ocean - carbonate readily forms wherever carbon dioxide and water interact, and on a world with carbon dioxide in its atmosphere (which should be any alien world capable of sustaining life-as-we-know-it), that becomes "anywhere there is water".
Carbonates tend to be slightly more soluble in weak acids than the other common biomineral salts (though it depends on the cation as well - magnesium carbonate is less soluble than calcium carbonate, for instance).
-
Calcite tests of foraminifers.
-
Cutaway of a nautilus shell, made of nacre - tiny aragonite plates layered on top of each other.
Oxalate
Oxalate (C₂O₄²⁻), another carbon-containing anion (structurally, essentially two carbonates joined together by replacing the double-bonded oxygen with a bond between the two carbons), is also found in biomineral salts on Earth, though typically only in minor roles - though, it may easily be far more important in alien life!
Oxalate, unlike carbonate, does not appear to be generated by inorganic processes; it is instead ubiquitously produced by plants (and a single genus of mold - Aspergillus), through incomplete oxidation of carbohydrates. It is, in fact, in human health, most notable for the form of biomineralization that occurs when consuming too much of foods high in oxalate/oxalic acid - the formation of kidney stones, which largely consist of calcium oxalate, as the oxalate bonds to calcium ions in your gut and forms solid crystal precipitates.
Oxalates tend to be somewhat harder and stronger than their carbonate equivalents, though not as much so as phosphates.
Phosphate
Phosphate (PO₄³⁻) is, perhaps surprisingly, not actually that common on earth, despite the fact that your very own bones are primarily composed of hydroxyapatite (Ca₁₀(PO₄)₆(OH)₂), and phosphate minerals of inorganic origin are quite common. Only vertebrates and the mantis shrimp (very specifically the mantis shrimp - no other arthropods) have been shown to have independently evolved phophate biomineralization; this leaves it having evolved only twice, compared to the dozens of times carbonate biomineralization has evolved.
The reason why phosphate biomineralization is so (evolutionarily) rare is not particularly well understood; it may be that the more complex crystalline structures of phosphates (relative to carbonates) do not occur to biological systems so easily, or that phosphate (itself a very essential component for life) is too precious to waste on structural material for most species, or some other reason. Regardless - this implies it is far from likely that alien organisms will have bones (or other mineralized components) made out of the same stuff as our own!
It is perhaps better to address the evolution of phosphate biomineralization not from the persepctive of why rather than why not? - it is, after all, far from the default, even if we tend to (in this case, erroneously) assume our own biochemistry's way of doing things is "normal". While there are multiple theories, one I've personally found convincing for how vertebrates evolved phosphate biomineralization is that it was deeply tied to the origins of vertebrate bone as a form of essential nutrient storage - not only is phosphate itself an essential nutrient, and storing it along with calcium a potentially useful adaptation in and of itself, primitive vertebrates would undergo periodic changes in the pH of their blood based on level of activity - changes in pH that might damage carbonate biomineral deposits, but which phosphate biominerals would be more resistant to.[3]
Phosphate biominerals tend to be dramatically harder and stronger than carbonates, and slightly denser and heavier.
Sulfate
Sulfate (SO₄²⁻) is yet another common anion in biomineral salts; substantially more common than phosphate, but much less so than carbonate.
Sulfate biominerals are, in fact, generally marginally softer and weaker than their carbonate equivalents, which one might imagine would make them worse as structural materials; one must wonder why organisms bother, when carbonate is so ubiquitous. The answer has to do with some of the particular chemical properties of sulfate.
For one, sulfates tend to be hygroscopic without being hydrophilic - they tend to absorb water from the environment, but don't tend to dissolve easily. This already makes them more viable in environments where carbonates are not, due to the relatively high solubility of carbonates; but it also allows a slightly odd capacity to tweak the properties of sulfate minerals via controlling their level of hydration. While sulfate is, itself, heavier than carbonate, and sulfate minerals therefore tend to be heavier as well (again, as shown in the case of strontium sulfate), highly hydrated sulfates will actually be lighter and less dense than carbonates; this means organisms can effectively "tune" the density of their sulfate biominerals over the course of their evolution, via the level of absorbed water present in the crystal structure, evolving them to be lighter or heavier as the organism adapts to different environments.
Second, sulfate tends to much more easily bind metal ions and precipitate out of solution than carbonate does; this makes sulfate biominerals generally cheaper, energetically, to construct, and makes them preferred for capturing relatively rare cations (such as the aforementioned strontium or barium).
Polyesters
Given that polyesters are typically thought of as synthetic polymers, it may be surprising to hear that polyesters do, in fact, occur and play structural roles in Earth life - although, in only two known cases, both of which are relatively marginal. In particular, the cutin that makes up the waxy outer layer covering the leaves of plants; and a special polymer secreted by bees of the genus Colletes to protect their brood cells. Both cases are, unfortunately, poorly understood - but they show, without a doubt, that polyesters are viable as a structural biological material, and there's no reason why alien organisms might not make much wider use of them! Some bacteria also make use of polyhydroxylalkanoates as an energy and carbon storage mechanism, but it seems to serve no structural purpose.
Polyesters tend to be divided into two categories, based on the motifs present in their chemical structure, which are, incidentally, also the two classes of hydrocarbon: aliphatic polyesters, where carbon atoms form open chains; and aromatic polyesters, where the carbon atoms form closed, six-member rings. Aromatic polyesters tend to be much more crystalline in structure (and therefore much harder and more rigid), while also being more chemically and thermally stable. Most are, however, rather difficult for organisms to synthesize.
While the most well-known synthetic polyester - and an obvious thought when hearing that polyesters are viable structural biological materials - is polyethylene terephthalate, otherwise known as PET, the conditions under which this is produced are not suitable for biological processes. There are, however, a number of other interesting polyesters to look at.
Polyesters from hydroxy acids
All polyesters that appear in nature here on Earth - although, this is a rather limited selection, given their relative rarity - are, it seems, derived from hydroxy acids (that is, carboxylic acids with at least one hydroxyl group), particularly short-chain fatty acids. The exact chemical mechanisms by which biochemical processes produce these polyesters is generally poorly understood, although polyhydroxylalkanoates are known to be produced via action of an enzyme on the hydroxy acids.
Cutin
Cutin, along with the related polymer suberin, is what provides plants with the tough, waxy outer coating of their leaves, assisting them in retaining their structure and making them hydrophobic so that rain slides off their surfaces. While it is not very well understood, it is thought to be a branched polymer of C16 and C18 hydroxy fatty acids, such as hydroxy palmitic acid. These very-long-chain acids, and its branching structure, result in an amorphous, soft, waxy structure, that is nonetheless fairly tough, completely insoluble in water, and highly hydrophobic.
Polyhydroxylalkanoates
Polyhydroxylalkanoates, such as polyhydroxylbutyrate, are another polyester that appears in nature - albeit not for structural purposes. It is used by a number of species of bacteria for energy and carbon storage. Nevertheless, it still provides an option to us for a structural biopolymer, as, on Earth, polyhydroxylalkanoates are routinely harvested from the bacteria that produce them and used to make strong plastics, particularly for biomedical use (as they are non-toxic).
As a structural material, polyhydroxylalkanoates are generally quite ductile, and may or may not be quite elastic depending on exact composition. They are tough and strong, with similar tensile strength to polypropylene. They are generally permeable to oxygen, but not to water. Compared to other polyester plastics, they also tend to have quite good UV resistance.
Phenolic acid polyesters
Phenolic acids - that is, a phenol with a carboxyl group, such as salicylic acid[4] - which are widely found in nature, have been demonstrated to be capable of forming polyesters under a variety of conditions. In the case that these are homogenous, linear polyesters (i.e. have no chain branches), their properties should generally be similar to other aromatic polyesters - that is, highly crystalline, and therefore hard and rigid.
More complex polyphenolic acids may instead more closely resemble polyphenols - indeed, phenolic-acid polyesters are both polyphenols and polyesters!
Polyesters from lactones
Lactones can be readily converted into polyesters via a variety of catalytic ring-opening polymerization reactions. While there are, as far as I am aware, no natural polyesters that are derived this way, lactones do occur in nature, and catalytic processes are well-suited for biochemistry.
Polylactic acid
Polylactic acid, most often known by its acronym "PLA", is a plastic most known, here on Earth, for its use as a 3D-printing filament. While it is not found in nature, it is catalytically synthesized from lactide (the lactone of lactic acid), which very much is, meaning alien organisms that synthesize it are highly plausible.
Unlike most other aliphatic polyesters, PLA ranges from semi-crystalline to highly crystalline in structure depending on exact composition, and is thus quite hard and rigid - it is somewhere in between polystyrene and PET in mechanical properties.
PLA is also sensitive to UV degradation, but this simply breaks off monomers, which could be biologically recuperated and the PLA repaired.
Polycaprolactone
WIP - needs more research
Polybutyrolactone
WIP - needs more research
Credit
Author: DocViviLeandra





