Conventional Guns: Difference between revisions
No edit summary |
No edit summary |
||
| Line 214: | Line 214: | ||
<td width=400>[[File:SimpFixedLeng.png|frameless]] | <td width=400>[[File:SimpFixedLeng.png|frameless]] | ||
<tr> | <tr> | ||
<td>Velocity developed by simplest gun with 300s propellant, plotted against charge to mass ratio, under conditions of various total tube length (barrel | <td>Velocity developed by simplest gun with 300s propellant, plotted against charge to mass ratio, under conditions of various total tube length (barrel is <math>\Lambda_u</math> times the chamber long when loaded to a charge-to-mass ratio of 1 or unity) and loading density. | ||
</table> | </table> | ||
Revision as of 08:40, 27 July 2023
Introduction
Conventional Guns denotes weapons which propels a mass (shot, projectile) using the exothermic decomposition of a chemical propellant (or charge) in a controlled manner, with propellant being constrained inside a barrel (as opposed to co-moving with the shot, as is the case with rockets). Having evolved from porcelain filled bamboo tubes that expel shrapnel using the explosion of gunpowder, attested to in the 12th Century in China, and subsequently introduced to Europe by the 14th Century, its prominence in literature that touches on military matters has been remarkable. Therefore it is not surprising that conventional guns continues to feature in many science fiction with a focus on warfare, although these depiction has seldomly been fair. Quite often, conventional gun technology have been treated as one of lower technological sophistication, that cannot hope to compete with other weapon systems (such as railguns, coilguns, and especially with missiles), and authors have often resorted to "too clever by half" twists that supposedly circumvent limitation of conventional gun, only to miss important caveats and leave out more plausible advances that may come either as natural consequences of the prevailing technological advancement of a setting, or as potential advances with some effort.
In any case, whatever the reason for this deplorable state of affairs may be, in line with Galactic Library's goal of providing literature where other sources fail, this article will seek to explore the design space of conventional guns with the help of interior ballistic theory, with a special focus on its impact for science-fiction worlds, and outline some real-world advanced techniques that promises to further improve upon this basis. Naturally, this will have particular impact on settings that are more near-future.
Theory of Interior Ballistics
Perhaps one reason for the general lack of interest, even in sci-fi interest groups, that conventional guns garner, can be attributed to the sheer complexity of the associated theory that describes the acceleration of shot under combustion of a propellant, known as the theory of interior ballistics. The nature of the process precludes simple and elegant equation like the analogous "Rocket Equation" for rocketry that encapsulates the essence of rocket kinematics. Instead, accurate models are always solved numerically. Thus this article will only go over the details of interior ballistics in so much as it lends to an intuitive understanding of the kinematics of gun systems, or as pertinent to establishing performance figures, and refer the reader to more systematic treatment of the same in reference section for the process of arriving at the detailed calculations later on. Notation in this article generally follows the Eastern tradition as established by M.E.Serebryakov, although the following argument has been much improved by consulting the work of J.Corner as well.
The Propellant
Propellants that are suitable for conventional guns must exhibit a stable burning (in this context, burning means exothermic decomposition in the absence of (appreciable) atmospheric oxygen) behavior. This is best illustrated by briefly going over the history of Nitrocellulose, the primary ingredient in almost all propellant up until rather recently.
When first synthesized circa 1845, Nitrocellulose was found to detonate violently, where upon ignition, a mechanical shockwave is rapidly setup, that propagates in all directions, compressing the material in front to the point of ignition as well. The shock front propagates at a few km/s, limited only by the (compressed) speed of sound in the combustion products, rapidly consuming all propellant. Thus, for 40 years, it was employed as an explosive, despite its advantage over black powder (thrice as much energy density, very little solid product and decreased sensitivity) being clearly evident. It was not until the mid 1880s that Nitrocellulose was tamed by dissolving it in a suitable organic solvent, such as alcohol or ether, that causes microscopic swelling of the micro structure of the fiber. In this state, the propellant behaves like a putty that can be extruded or cast to the desired shape. This leaves microscopic holes, or pores after the solvent is driven off through heat. Now, the behavior of burning changes completely: When ignited, the propellant is heated, at the surface, by the radiated heat of the exothermic combustion products. This causes burning to (largely) happen along a parallel plane to that of the initial shape, with the rate limited by the bulk thermal conductivity of the propellant, to around a few cm/s, at gun pressures, a reduction of five orders of magnitude. This development allowed Nitrocellulose to rapidly replace black-powder as the preeminent component in gun propellants in the next decade, relegating the latter to be used as an igniter and ignition booster for the most part.
A brief discussion of further development of propellant science since that period to that of modern time is given for the sake of completeness, to spare the reader of tracking down fragmentary information across multiple, cryptic DTIC reports and digging through Internet Archive.
In the decades immediately following, Nitroglycerin was added to the mix and the propellant is dissolved in the common solvent Acetone, greatly increasing both the energy density and flame temperature (and errossiveness) of propellant. This being known as the double-base propellant, the original formulation became retroactively known as single-base propellant. In the wake of WW1, the German innovation of using a Nitroglycerin - Ethyl Centralite mixture as both a component of the final propellant and a solvent to cause swelling, eliminating the time and expense necessary to extract the solvent. This "solvent-less" gun powder was rapidly adopted, and especially useful in rocket motors where it would have been extremely difficult to extract solvent due to the thickness of the cast involved. Immediately prior to WW2, another German innovation, that of a modified double base propellant where Nitroglycerin was replaced in part or in full with Diethylene Glycol Dinitrate (DEGDN), both relieved the war-industry of the burden of Nitroglycerin production (which depended upon foodstuff) while also improving the thermal-chemistry of propellant gasses, with overall lower molecular weight, facilitating either increased force or reduced temperature. The downside is reduced thermal stability, much noticeable in hotter climates. Triple base propellant, involving the addition of Nitroguanidine, as the primary energetic component, increasing the performance further, although the higher cost (and limited supply) of Nitroguanidine delayed its adoption until after WW2.
As of late, modified double-base propellant and triple-base propellant appears to be the choice when performance is required of gun system, and expense can be spared in pursuit of the same, while single-base propellant remains dominant for small-arms purposes, or when a certain measure of economy needs to be exercised. Reflecting their usage, the former two usually comes in grains of defined shape and perforation, sized to suit their gun, while the latter tends to be extruded or grinded down into simpler geometry like strand or ball, in standardized dimensions.
Although attempt has been made since then to replace triple base propellant with Mixed Nitrate Ester propellant of greater availability, and indeed various mixture had been type classified, composed of Nitrocellulose mixed with varying proportions of BTTN, TMETN, TEGDN and DEGDN, this appears to not have been widely adopted, perhaps due to a lack of necessity. Finally, it bears mentioning the composite propellant, consisting of a heterogeneous mixture of oxidizer and fuel particles. This appears to not have been adopted for internal ballistic use as much as it is prevalent in rocketry (various Aluminum Percolate Composite Propellant, or APCP comes to mind), due to its lower performance than pure CHON systems.
In recent years, two of the more promising lines of research that bears special mention includes the work on Nitramine propellant, and that of highly energetic poly-Nitrogen Compounds (otherwise colloquially known as Nitromemes in relevant interest circles). The premise behind Nitramine propellant is simple enough: that of taming RDX (Hexogen, Cyclonite) and HMX (Octogen), well known and highly energetic chemical explosives, for use as propellants. It was found that by grinding RDX particles down to 5 micrometers or so, and suspending these particles in plasticizes, these energetic materials could be made to combust stably enough for use as propellant (specifically, this brings the burn rate pressure exponent below 1). RDX based propellant development effort has already bore fruit, with formulations being type classified by the 1980s to 1990s, while HMX based propellant is still under investigated. Work on denser, and even more chemically favorable polycyclic nitramines like CL-20 (also known as HNIW) based propellant is well underway as well.
Another, more speculative line of research concerns poly-Nitrogen compounds. While Nitrogen allotropes, whose names grows in exoticism with the number of atoms being forced together, from Trinitrogen (N3) and Tetranitrogen (N4), to the likes of Hexazine (N6), Octaazacubane (N8), and Bipentazole (N10), are generally considered too unstable for use as propellant (indeed many aforementioned species are considered meta-stable with lifetime measured in seconds). If these can be made to be somehow stable in storage, and dissociate at a reasonable rate after ignition, the much higher density of energy stored within coupled with reasonable molecular weight (the primary product will be N2 (gas) at ~28, as compared to 23-25 of current propellant) would expectantly produce gun system of tremendous performance. However, there is no research at present hinting at how this might be achieved. As a concession to practicality, less energetic compounds can be had with the addition of C,O,H,F to high enthalpy Nitrogen, creating chemically stable structures. It is possible that with the use of inert binders and by applying similar techniques to that of stabilizing Nitramine propellant discussed above, these could be made to burn at reasonable rate, although this too remains in the realms of speculation.
Five propellant properties are relevant to interior ballistics. A brief technical discussion of these is given here. A few assumptions bears mentioning. First, it is usually implied in ballistic work, that the propellant being treated is chiefly a system of equilibrium involving Carbon, Oxygen, Hydrogen and Nitrogen. In this system, the most important reaction at relevant conditions is the water-gas balance (CO + H2O <-> CO2 + H2), therefore peculiar properties of this reaction forms the basis of certain shortcuts we take to simplify our analysis. Second, we have assumed that the properties of gas is constant throughout its entire time in bore. This is analogous to the concept of "Frozen Flow" in rocketry, where detailed theramlchemical calculation is done for "representative condition" (or at best a few conditions, taking an appropriate average), and the gas is taken to not deviate too much from equilibrium. This approximation is justified by working out the reaction rate of the chemicals involved, which is so fast for conditions near the peak pressure point that equilibrium is almost guaranteed, and quickly drops off as the gas cools to below 2000K, at which point reaction almost completely ceases. This also prevents the formation of re-combination products like Methane (CH4) that would otherwise have greatly impacted the result. Of course this is merely a general trend, and calculations can always be improved by incorporating these effects, with diminishing return on effort invested.
- Propellant Force , also known as specific impetus, measured in unit of energy per unit of mass. This is the analogous property, in ballistics theory, to the concept of specific impulse in rocketry. Unfortunately, it has a more tortuous definition of either "the difference between the enthalpy and energy of gas product at adiabatic isochoric flame temperature for one unit mass of propellant burned", or equivalently, "the work done by an unit mass of propellant ideal gas expanding in an adiabatic and reversible way from the adiabatic isochoric flame temperature down to 0K". By definition, propellant force is:
In practice this value is usually derived from peak pressure readings from closed-bomb test according to ballistic theory, with an eye towards representing the condition encountered in its application, which differs slightly from that predicted by thermal-chemical balance calculations. By assuming ideal gas relationship (i.e.), this allows relating the (ballistic) propellant force to the (rocketry) specific impulse via: - Bulk Density , or the density of propellant formulation. For most propellant in use this value is around 1.6 g/cc, although propellant with higher density (CL-20 @ 2.0g/cc) is not unknown.
- Covolume , measured in the inverse of density unit, is found in the Nobel-Abel equation of state commonly assumed for interior ballistics work:
In practice this is usually fitted to a closed-vessel test fire data, making it really an average over the range of condition experienced by gas in chamber. Since, in fact covolume is dependent on chamber loading condition and pressure developed, it is fortunate, then, that its effect is most prominent during the starting phase of ballistic cycle where gun pressure are the highest, and in that range of conditions covolume typically only changes by less than one percent. And although the variation is greater at conditions below that, the contribution is much less, as the effect of covolume is less pronounced at lower pressure in any case. As a result, the experimentally determined value is in agreement to within a few percent of that calculated from the more sophisticated (but developed much later) Virial equation of state, taking the first 3 Virial coefficients and using the highest density encountered.
For almost all propellant currently used the covolume lies very narrowly in the range of 0.9-1.1 cc/g. - Burn Rate Coefficient and Burn Rate Exponent . The dependency of burn rate upon reaction zone temperature and pressure is a complex topic.
By examining partially burnt propellant through specially vented chambers, it was established in the 19th century that propellant burning proceeds parallel to the surface of a propellant grain, or in other words, at any time the surface of burnt propellant roughly parallels the surface it had before burning. This observation is known as the Piobert's Law (see above for an explanation), gives rise to the definition of "propellant (linear) burn rate" as the rate at which this parallel surface recedes.
It is now known that propellant burn rate exhibit several distinct "zones" with each range exhibiting different temperature or pressure dependency, especially in the low pressure ranges (~few tens of MPa). Historically, due to the limitation of instrumentation these idiosyncrasies were not known to a reasonable degree of accuracy, ballisticians have resorted to fitting burn rate to pressure in the range where accurate representation is most important, i.e. at high pressure and close to the operating pressure of guns. By far the widest used representation, is:
These two properties are perhaps the least explained of all as propellant performance is concerned. While empirical rules have been developed to describe the burn rate of arbitrary mixtures of some well known propellants , and it is possible, through very detailed calculation, to predict the burn rate of a propellant, this is in practice seldomly done unless very specific features of combustion are being studied. Uncertainties in these areas are also the primary concerns for prospective and hypothetical propellants, since these generally require enough quantities to be synthesized for testing in a closed vessel, and even then sometimes data are only available for very low pressures due to the scarcity of high-pressure closed vessel apparatus capable of performing such experiments.
A brief note on the techniques for estimating these parameters. Recall the water-gas balance (CO + H2O <-> CO2 + H2). It was noted by Hirschfelder and Sherman in the 1930s that, not only is this reaction not excessively influenced by pressure due to having a balance of gas molecules on either side, as mentioned above, but also by a peculiar coincidence of numbers of the mean molecular weight and heat capacity, the propellant force is also relatively insensitive to the ratio of CO2 and H2O. This, then, allows estimation to be made with only the ratio of atoms present within a CHON system, without regard to solving the exact equilibrium and assuming a priori a ratio, of almost all the thermal chemical properties relevant to ballistics. Other properties like covolume can then be fitted to these estimated properties. This is the premise of the Hirschfelder-Sherman estimation technique, whose result are usually within 2-5% of the far more laborious solution.
Otherwise, it is necessary to use the more conventional method that rocket scientists have been perfecting for the better part of a century, with special care of using constant-volume thermal properties instead of the constant pressure ones usually given in relevant databases. By iteratively calculating the chemical equilibrium giving an assumed reaction temperature, calculating the difference between the heat of formation of the product and their predecessor, adding in the internal energy of the reaction product, and revising the temperature estimate based upon this, it is possible to, finally, rigorously estimate these up to the accuracy of the underlying database. This method is detailed in almost every standard interior ballistics textbook and is also used to generate the tables present in this article. (WIP)
The Simplest Gun
The simplest gun system in terms of ballistics theory is one where all the propellant has burned to depletion before the shot has appreciably moved. The shot is then propelled by adiabatic expansion of high pressure combustion product down the bore. An additional simplification introduced here is that the weapon does not have any chamberage (i.e. the chamber is of the same diameter as the barrel, see later for an discussion about chamberage effects). This is hardly a realistic proposition for a practical weapon, as this type of gun would demand excessively heavy and thick chamber to contain the pressure developed in what amounts to the isochoric combustion of the propellant gas. Instead, the value of this idealized representation lies in its simplicity. By ignoring the minutiae of burning, it eliminates most theoretical difficulties and reduces the problem of interior ballistic to its central problem: namely, under the constraints of:
- The gas velocity, for a small group near the breech face, must average 0.
- The gas velocity, for a small group near the bottom of the shot, must average the shot velocity.
determine the distribution of group velocity, pressure and density along the barrel. This problem, named after the great polymath Lagrange that first formulated and attacked it, is often simply called the Lagrange problem in interior ballistics. Various attempts has since been made by postulating some initial distribution of
The Lagrange Approximation
Lagrange, having formulated this problem in 1793, attempted to simplify it by introducing an additional assumption, that the gas density is constant throughout the entire volume. The implication of this is explored more fully by Piobert and later Sebert (late 19th C.). Substituting into the equation of continuity:
Using Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle v_g} to denote the velocity of gas. Noting that
Integrating on both sides yields:
Implying the gas velocity is linearly distributed along the length of barrel between the breech face and the shot base. It then follows that the pressure is distributed in a quadratic manner along the gas volume:
Where denotes gas pressure at location along the barrel, the pressure at shot base (s stands for shot), and the resistive pressure acted upon the shot, respectively. is the shot travel at that point. It is also customary to express drag simply as a ratio of shot base pressure: . In particular, the breech pressure when taken at is:
Evaluating the integral , the space-averaged pressure is:
The ratios , that appears in the above equations are known as Piobert's constants, values whose physical meaning will be explored in the later paragraphs.
The length we have came to discussing the specifics of the Lagrange approximation is due to the remarkable agreement with reality. It is now generally accepted that the assumption of uniform density is enforced by the presence of pressure waves that reflects between breech face and shot base, tending towards equalizing the density along its way, towards the uniform value that the Lagrange assumption presuppose. Of course, this only explains how a condition of uniform density might be developed, without regard to the actual initial condition of the gas, which motivated others to examine more "physically plausible" configurations that can naturally develop, see below.
From this, we can also glimpse the limitations of Lagrange approximation. As the charge to shot weight ratio increase, it can be shown that these pressure waves (if there were to be such disturbances) would become increasingly rare, spending most of the time the shot is in bore catching up to it, with the gas exhibiting considerable difference of density along the volume. Then, the Lagrange approximation cannot be thought of as appropriate.
The Pidduck - Kent Approximation
While the Lagrangian approximation is useful as far as practical applications are concerned, there is some interest in developing an approximation that is plausible, that is it naturally derives from certain initial configuration. The Pidduck approximation is really the culmination of a long line of work by Riemann, Hugoniot (1889), Gossot and Liouville (1914), and Love and Pidduck (1921-1922). The premise is simple enough: the pressure, density and temperature are all uniform, and gas behaves adiabatically. Then, rarefaction waves that appears as the shot starts moving are laboriously traced as they refract, finally leading to a pressure distribution at any time behind the shot, the exact solution of which strained the computational capabilities of the time to such an extent that only certain special solutions were attempted. A feature of note is that no shockwaves were predicted in these solutions.
It was noticed by Pidduck (1936) that these initial pressure waves are damped out rather quickly, leading to convergent solutions for when the shot has traveled appreciably. Pidduck's limiting solution refers to one such solution. Kent (1936,1937,1938) derived essentially the same limiting solution via a different method, leading to the solution being associated with both in Western academic sources, although the name of Pidduck is more recognized in Eastern literature.
Since even Pidduck's limiting solution is still rather involved, the details are elided here and the reader is referred to standard textbook in interior ballistics for details. The limiting solution is strikingly similar to that of the Lagrange, as a velocity distribution approaching linear is predicted, although the pressure and density distributions are much complex.
This idea is further developed by M.A.Mamontov (1959), noticing that among others, Pidduck's limiting solution does not actually depend on inclusion of the covolume factor. He instead attacked the problem under the assumption of a uniform temperature field, as it was felt that at higher ratio of charge to shot, it is least unlikely that temperature remain relatively constant, as compared to any other parameters being studied. His solution is now understood as the limit of Pidduck's limiting solution with as adiabatic index approaches unity. Being developed in the Soviet Union, his work is relatively unknown in the West as it was felt that this line of inquiry has been exhausted on a high note with Pidduck and Kent's work.
The closeness of various approximate solutions to the Lagrange problem can best be appreciated by listing the respective Piobert constants for each.
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
It must be stressed that the agreement of multiple theories does not imply agreement with reality. In fact, the theoretical justification for all approximations detailed above are strongest when charge to shot weight ratio is small, disagreeing with each-other (and with experiment) as increase. Experience has demonstrated this agreement as "excellent" below 0.5, and these have been applied in literature, apparently without fanfare, to modern APFSDS rounds whose value approaches unity.
Solution for the Simplest Guns
Many similarity relations exist in the study of interior ballistics. For the simplest gun, having determined the propellant, only few parameters are required for establishing its performance. These are:
- Effective Charge to Shot Mass Ratio, .
- Loading Density, or , the mass of charge divided by the total chamber volume. It bears mentioning at this point that for interior ballistics purposes it is customary to ignore any air trapped in chamber as air density is considerably lower than that of the propellant's density, even accounting for the inefficiencies of packing a geometrical shape within a tube. This is usually taken to be around 50% to 60% of the bulk density of a propellant, or around 0.5-0.7 g/cc for large caliber weapons and approaching 1g/cc for small arms.
- Barrel Expansion Ratio, or . Since we have chosen to neglected the effect of covolume, this is simply where . and are the barrel volume and chamber volume, respectively, while and denote the length of gun to the length of chamber, finally with the cross sectional area of the barrel.
It should be fairly evident that if the gun solution can be expressed in these parameters, by examining the variation of performance with these parameters one can generalize the findings by substituting in the specific values for shot mass, loading density and of barrel expansion. For the following analysis, the ballistic solution of a hypothetical propellant of s, , g/cc, and cc/g will be considered. These are rather standard values for propellant used today.
Now, it is finally time to tie in all that we have discussed and examine the problem of the simplest gun. The initial condition (as far as the shot is concerned) has the propellant fully combusted, and since the shot has not appreciably moved, the volume the combustion product occupies is precisely the chamber volume. The gas temperature is then exactly , as defined. Substituting the relevant conditions into the Nobel Abel EoS gives:
Since there are no further addition of gas behind the shot, and as the shot starts moving, the pressure can only decrease, it is appropriate to label this point both as both the initial value (subscript 0) and the maximum value (subscript m).
As the shot starts moving, not only does the volume increase, but also some energy is extracted from the propellant gas, accelerating the projectile.
Recall from our discussion of the Lagrangian problem and especially its approximations, that a constant (for this particular loading condition) ratio between the space mean pressure and shot base pressure exist. It stands to reason that since work is but force integrated over distance, and that both gas and shot starts with no (macroscopic) kinetic energy, this would cause the kinetic energy of the two to be related. Indeed it can be verified through the use of calculus (this assertion is usually left as a homework for students of interior ballistic courses since it involves only rote application of conventional integration rules), that the total kinetic energy of the shot and gas can be expressed as:
- where
Now, noting that the energy must be conserved between the internal energy of the gas, the kinetic energy of the gas, and finally the kinetic energy of the shot. For ideal gas, the specific heat at constant volume, , is:
- , with
In fact, this is a function of temperature. It is possible to choose, through an appropriate averaging procedure, to adopt a "gun gamma" for this purpose, although even this must be corrected with empirical correction factors. Nevertheless, proceeding with this simplification, this allows us to rewrite the equation above to be more general:
Noting the effect of covolume, the gas relation for adiabatic expansion can be rewritten as:
With these equations it is now possible to solve for the relation of , and , though in a rather roundabout manner. Rearranging after Corner, this gives:
This can either be interpreted as predicting the velocity of shot for a gun (with unspecified length) at a certain point , or the muzzle velocity of a gun that is of length , then the factor in the above is simply , finally reducing the solution for the simplest gun to the three parameters under study, and properties of the gun propellant chosen.
It should be noted at this point that the factor is simply the expansion ratio (volume behind shot to chamber volume) after accounting for covolume effect, so that loading density and barrel expansion ratio need not be discussed separately. With that fixed, the velocity developed increase with the square root of charge to mass ratio at any given barrel expansion ratio.
With that in mind, the achieved muzzle velocity from the aforementioned hypothetical propellant can be graphed with respect to the charge to shot mass ratio as such:
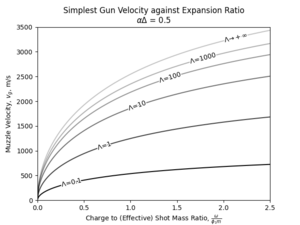
|
| Muzzle velocity developed by the simplest gun using the hypothetical 300s propellant, with a loading density of 0.5g/cc at various barrel expansion ratio. |
In the range shown, the velocity developed by the gun at a certain charge-to-mass ratio increase, with rapidly diminishing return, up to a certain limit. This limit is found by:
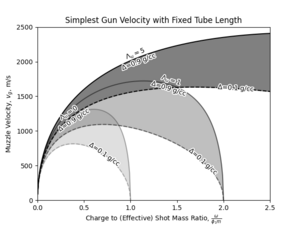
Another way of considering the above relation is to start with a fixed total "tube length", and explore the influence of loading density and barrel expansion ratio on the velocity developed at muzzle. In this scenario, both the chamber and the barrel volume are variables dependent on the design parameters we have chosen. Then, the results are as follows:
The appearance of optimal charge to mass ratio for a given combination of total tube length and loading density conditions can be explained by the balancing effect of increased charge also reducing the effective expansion rate by reducing the free volume in the barrel. At higher loading conditions this appears to occur at higher charge to mass ratios.
(WIP) It can be shown that this type of gun achieves the highest possible ballistic efficiency (the amount of energy transferred to the shot out of the total energy content of the propellant load) out of a given barrel length, given a certain weight of propellant and shot.








![{\displaystyle I_{sp}\cdot g_{0}={\sqrt {{\frac {2f}{\gamma -1}}[1-({\frac {P_{e}}{P_{c}}})^{\frac {\gamma -1}{\gamma }}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/289d0d1f6d1fad3bae85a2580157f19514458c29)


















![{\displaystyle P_{x}=P_{s}+{\frac {\omega }{2m}}[1-({\frac {x}{l}})^{2}]\cdot (P_{s}-P_{r})}](https://wikimedia.org/api/rest_v1/media/math/render/svg/22368d3f322766d81dabc56d34dc6e0828a4051e)

































![{\displaystyle P(l,v)={\frac {f\omega -{\frac {\theta }{2}}\varphi mv^{2}}{S[l+l_{0}(1-\alpha \Delta )]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/797f2b2e14ae8be193b2515dc8d5c9bd7076ae76)