Structural Biological Materials
Real organisms use a wide variety of structural materials to give toughness, strength, rigidity, and other desirable material properties to their bodies, both on the cellular (as in cell walls) and tissue (as in bones or exoskeletons) level. Moreover, these materials are rarely homogenous - biological structural materials are almost always composites of more than one material, becoming something greater than the sum of its parts.
Rather than simply take the approach of listing options, this page will aim to explore the general categories of structural biological materials - both real and theoretical - and explain how their particular properties play into how they're used in living creatures.
Polysaccharides
Polysaccharides are perhaps the most widespread - and simplest to evolve - structural materials we find in living creatures. Saccharides, especially glucose, are universally used as energy storage in Earth life, and it's likely that alien life - at least, that which shares our basic biochemistry - will do the same. It therefore isn't much of a step to derive your structural materials from the stuff you already use for energy storage!
Even though monosaccharides are typically hydrophilic, and readily dissolve in water, most polysaccharides are, in fact, quite hydrophobic and insoluble. This makes them quite well-suited for assembly-on-the-spot - the component monosaccharides can be dissolved in the cellular medium for transport, and will precipate out of the water as they are assembled into polysaccharides.
One defining element of polysaccharides, on the chemical level, is the multiple free hydroxyl groups on each monomer of the chain. Through a variety of processes, these can be replaced by other functional groups, altering the properties of the polysaccharide; they may also serve as useful bonding sites for other structural molecules, such as peptides and polyphenols. This chemical versatility is another reason why they are so ubuiquitous in Earth life - they excel at a wide variety of structural purposes, and make a very good "foundation" for other structural biological materials to build on in composite materials.
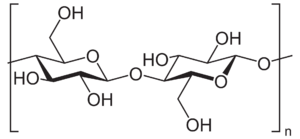
Cellulose, and other simple linear polysaccharides
Cellulose is the simplest and most widespread polysaccharide that sees structural use on Earth, and is likely to be so on alien worlds as well. It's a simple linear chain of β(1→4) linked units of d-glucose - the same molecule nearly all life on Earth uses to store energy. Aliens might use ʟ-glucose instead, but this should have identical mechanical properties.
Contrary to popular belief, cellulose is actually quite flexible, when alone. The rigidity of plant cells - famously with cell walls made out of cellulose - is provided by other materials bonded with cellulose, such as lignin, along with the hydrostatic pressure provided by the water within the cell.
A number of Earth organisms are demonstrated to use mannose, or other simple sugars, in the place of glucose, forming structural polysaccharides such as mannan. Their properties are similar to cellulose - though, how they may bond to other polymers might differ, as we'll discuss later with glucomannans.
Some other organisms, such as oomycetes, use other β-glucans - that is, linear chains of glucose - which have different linkages (such as the β(1→3) linkages of chrysolaminarin) as structural materials. In bulk, their mechanical properties are fairly similar.
Hemicelluloses - heterogenous simple polysaccharides
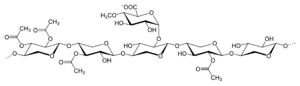
In plants, cellulose is not found alone - it is typically accompanied by filaments of hemicellulose, otherwise known as polyose, a group of highly heterogenous polysaccharides composed of a semi-random mix of miscellaneous saccharide molecules - predominately arabinose and xylose. The heterogeneity of these fibers inhibits the formation of crystalline structures the way cellulose might form, rendering the result relatively amorphous and weak: if isolated, hemicelluloses would have a gum- or gel-like texture. While that may seem undesirable in a structural material, hemicelluloses perform important functions in plants - in particular, cross-linking cellulose fibers together, adding additional strength and toughness to the resulting matrix.
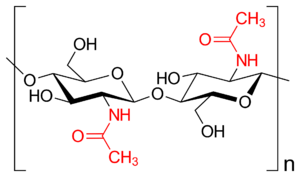
Chitin, and other poly-amino-sugars
Chitin is the second most widespread structural polysaccharide on Earth, and for good reason. Like cellulose, it is a linear chain of single monomers. Rather than being made of simple glucose, however, it is composed of N-acetylglucosamine - an amino-sugar of glucose where one of the hydroxyl groups is replaced by an acetylamino moiety. This acetylamino moiety forms stronger hydrogen bonds with adjacent polymers than the hydroxyl group would, rendering chitin much stronger and slightly more rigid than cellulose - though, it still remains plenty flexible, without other components to rigidify it.
N-acetylmannosamine and N-acetylgalactosamine, derivatives of mannose and galactose, respectively, also exist in nature - to my knowledge, they are not used to form polymers like chitin, but an alien organism may do so!
Hydrophilic polysaccharides
Though most polysaccharides are hydrophobic, this does not apply to all. Many are possessed of an overall negative charge and/or are internally polar, allowing them to become quite hydrophilic. As hydrophilic molecules, they will readily dissolve in water - a characteristic exploited in certain biological lubricants, such as the fluid that fills the space between joints in vertebrates, which consists of relatively short chains of hydrophilic polysaccharides dissolved in water (when you pop your knuckles - or other joints - what you're doing is popping bubbles in this fluid).
However, though these polysaccharides dissolve in water, this does not break the bonds of the polymers. This is a property of water-soluble polymers in general - though they may "dissolve", they do not break apart. This means relatively long polymers, especially those with extensive cross-linkages between them, will maintain their structural form when hydrated - instead, the water infiltrates the polymer structure, causing it to swell into a sort of hydrogel or gum, which may be stiffer or softer depending on how closely interlinked the polymer chains within it are. Water can also pass through the polysaccharide gel, at a rate which is, again, dependent on the exact structure of the gel - which may assist organisms, particularly unicellular or colonial ones, in osmoregulation. This structural usage of hydrophilic polysaccharides is obviously better suited for aquatic biota, which do not have to worry about their polysaccharide hydrogels drying out - but can still be quite useful in the interior structures of terrestrial biota.
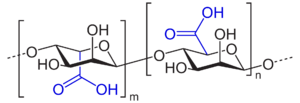
Algin, and other polyuronic acids
Algin, or alginic acid, a polyuronic acid, is a primary structural component of the cell walls of brown algae, such as kelp. It is a polymer of two uronic acids in alternating sequence - β-D-mannuronate and α-L-guluronate. Uronic acids are derivatives of saccharides where the hydroxyl group opposite the carbonyl has been oxidized to a carboxyl group, making them a kind of carboxylic acid. This makes polyuronic acids acidic (as implied by the name) - which in turn makes them hydrophilic and water-soluble.
The hydrophilic nature of algin is responsible for the gummy, gel-like texture/structure of the brown algae.
The carboxyl group replacement that defines the uronic acids very rarely co-occurs with replacements of other hydroxyls on the same monomer with other functional groups, for reasons I don't personally understand. For example, I know of only one amino uronic acid - N-Acetyltalosaminuronic acid.
Sulfated polysaccharides
Sulfated polysaccharides are common throughout Earth life, and should be expected to be common among alien organisms with broadly similar biochemistry. In sulfated polysaccharides, one or more free hydroxyl groups are replaced by a sulfate ion; in some cases, sulfates occur only once every several monomers, while in others, nearly every single hydroxyl group in the entire chain is replaced by a sulfate.
Like polyuronic acids, sulfated polysaccharides are hydrophilic, due to the negative charge of the sulfate ion. However, they have a range of properties which distinguishes them profoundly from their uronic acid equivalents.
First, whereas the carboxyl groups that define the uronic acids rarely co-occur with other hydroxyl-replacements on the same monomer, sulfation frequently occurs with other functional group replacements - particularly, sulfated amino sugars, where one or more of the remaining hydroxyl groups (after the addition of the amine) has been sulfated, are quite common in nature, allowing a single monomer to bear the characteristics brought by both sulfation and other derivative processes. This extends to, as previously mentioned, molecules where each monomer has had all free hydroxyl groups sulfated, resulting in a much greater negative charge (and thus, greater hydrophilicity) than in uronic acids.
Second, sulfated polysaccharides are quite good at binding and precipitating metal ions, such as calcium or sodium, out of solution. Relatively short sulfated polysaccharides, therefore, often play a non-structural role in regulating the transport of ions throughout an organism; and sulfated polysaccharides in the outer surfaces of aquatic organisms, such as in the cell walls of many algae, may assist in the capture of necessary ions from the water. However, this also lends itself to a structural purpose - sulfated polysaccharides appear to play a role in biomineralization in some clades, such as in the coralline algaes, precipitating metal ions out of solution to form hard, mineralized coatings on the organism's exterior.
Disaccharide polysaccharides
As demonstrated by algin, above, many structural polysaccharides, rather than consisting of a homogenous chain of a single monosaccharide molecule, are instead often composed of two distinct monosaccharides in alternating sequence. This allows the copolymer to have some of the properties offered by both monosaccharides.
Glycosaminoglycans
Glycosaminoglycans, also known as mucopolysaccharides, are a common form of alternating polysaccharide, consisting of alternating units of a uronic acid and an amino sugar, the latter of which may be sulfated (as in heparin) or not (as in hyaluronan). These polysaccharides are very polar, and attract water very strongly as a result, while also self-adhering quite well.
Glycosaminoglycans fulfill a variety of important functions, particularly in animals. Since diffusion through them is possible, but quite slowly, they help slow and regulate the intercellular diffusion of substances like ions and proteins; since they can adhere quite well, they serve as an adhesive binding different cells together; and since they attract water so well, they are used to assist in the retention of moisture where watery surfaces must be exposed, such as in the eyes of vertebrates.
Peptidoglycan
Peptidoglycan, also known as murein, is the primary structural component of bacterial cell walls. It is an alternating copolymer of N-acetylglucosamine (as found in chitin) and N-acetylmuramic acid - a complex monosaccharide where a lactic acid moiety has replaced yet another hydroxyl group on N-acetylglucosamine. Like in chitin, the acetylamino moieties serve to assist in the formation of crystalline structures through strong hydrogen bonds; the addition of the lactic acid to every other monomer in the chain, however, serves as a strong binding site for short oligopeptides (consisting of three to five amino acids) which bind to a different polysaccharide chain at each end, forming a regular mesh that resembles a chain-link fence.
The resulting structure is very hard to break apart chemically, and strong enough to maintain the regular shape of cells observed in most bacteria. There is no particular reason, in fact, why it is not found in eukaryotes or archaeans, other than these two groups simply not having evolutionarily stumbled upon it - peptidoglycan only evolved once. So, complex alien life might use it!
Branching polysaccharides
As seen above, almost all polysaccharides used for structural purposes in living organisms are linear chains, though branching polysaccharides are commonly used for energy storage. However, some branching polysaccharides do, in fact, see structural use - such as the glucomannans found in some yeasts and plants. Glucomannans consist of a linear chain of mannose units (called a mannan), with short glucose branches. Unlike linear polysaccharides, branched polysaccharides resist forming neat, crystalline structures (as the branches interfere with hydrogen-bond formation between polymers), and thus are able to slide past each other, forming a rubbery, hydrophilic solid, or even a viscous liquid (when hydrated).
Polyphenols (lignin, tannin, etc.)
Polyphenols, such as lignin and tannin, are a broad category of biopolymer found almost exclusively in plants on Earth, consisting of many phenol units oxidatively coupled together. Unlike other common structural biopolymers, polyphenols are typically non-linear - instead forming complex 3-dimensional structures. They are also often highly heterogenous, made of many different monomers. This makes it difficult to talk about sub-types of polyphenol, as we have done for polysaccharides - instead, this section will talk about the properties different polyphenols often possess.
Color
Polyphenols' complex 3d structures leads to a variety of interactions with light waves that result in them being typically opaque and strongly colored. While most polyphenols are dark brown - lignin being responsible for the dark brown color of most tree bark - some polyphenols have brilliant colors, such as the anthocyanidins that provide flower petals with vivid reds, purples, and blues.
While structural polyphenols are typically quite separate from pigment polyphenols, an organism that is able to synthesize one is likely capable of synthesizing the other!
Reactivity
Whereas most structural biopolymers are only weakly reactive, polyphenols are quite reactive, specifically to oxidation. This might sound like a vulnerability for a structural material, but polyphenols' reactivity is usually localized to a few specific sites on the molecule. This makes them very good at binding to other structural polymers, like polysaccharides and proteins, at multiple sites, via oxidative coupling. Other structural biopolymers bound by polyphenols in this way tend to become exceptionally hard and rigid - like tree bark.
Resistance to degradation
Some - not all - polyphenols, such as lignins, are exceptionally resistant to bio-degradation, making them very difficult for other living things to break down and digest - and this extends to the other polymers they bind to. This makes them very good at protecting from penetration by parasites or pathogens, and limits the possible predators of organisms that use polyphenol cross-linking in their structures.
Affinity for saccharides
Polyphenols have something of a special affinity for saccharides, and will not only readily bond with them, but also often include one or more saccharide molecules in their structure - such as in tannic acid, a polyphenol composed of phenol branches stemming from a glucose molecule at its center.
Cross-linking proteins (collagens, keratins, sclerotins, etc.)
Proteins - perhaps unsurprisingly, given their ubiquity - are common components of structural biological materials, specifically in the form of cross-linking proteins like keratins or sclerotins, which bond to each other at many different sites, allowing for not only toughness and rigidity but a tunable degree of both - via controlling the frequency of cross-linkages, materials can smoothly vary in rigidity and hardness, often forming distinct gradients in biological structures (such as arthropod cuticle becoming more flexible around the joints).
Most cross-linking structural proteins tend to be brown to black in color, though are not particularly opaque, and some can be outright colorless. Their versatility also lends themselves to the creation of complex microstructures resulting in vivid structural colorations, and tissue that includes them often also plays host to a variety of pigments that add their own colors to the material.
Collagens
Collagens are the primary family of structural proteins found in all metazoans, though they are most prominent in the deuterostomes - in protostomes, many of the functions typically performed by collagens are instead performed by chitin. In mammals, collagens make up 25-35% of all proteins in the body! They make up the connective tissue, while also adding strength to other tissues (such as blood vessels) throughout the body, and also providing the matrix on which hydroxyapatite is deposited to form bone and cartilage in vertebrates.
Collagen consists of a left-handed triple helix of elongated peptide chains. About half of its amino acid content is glycine and proline alone, which appear to play a role in binding the three components of the helix together. In addition, sites throughout the chain are saturated in hydroxylysine - a hydroxylated derivative of the amino acid lysine. It is by these hydroxylysine sites that collagen helixes are cross-linked - via aldol condensation, strong covalent bonds are formed through conjugated enone moieties between the hydroxylysine sites on adjacent collagen helixes. This binds the adjacent helixes together very strongly, while still allowing hydrolysis of the linkages when they must be split apart, unlike certain other structural proteins.
Unlike most structural proteins, collagen appears colorless, or sometimes white. It is also extremely flexible - collagenous tissue must rely on other components to add hardness and rigidity, if necessary.
Keratins
Keratins are the primary family of structural proteins found in vertebrate integument - composing hair, nails, feathers, scales, etc, while also reinforcing the epithelial tissue (the outermost layer of the organs and blood vessels). There are two primary categories of keratin - α-keratins, which are found in all vertebrates, and β-keratins, which are found only in sauropsids. The primary difference between these is that α-keratins form double-helix filaments and are more flexible, whereas β-keratins form β-pleated sheets and are significantly harder and more rigid.
Both types of keratin crosslink by means of disulfide bridges - they contain large amounts of the amino-acid cysteine, which bonds to cysteines on adjacent keratin polymers via the sulfur. These bonds are very strong and rigid, and, as mentioned before, the precise degree of hardness and rigidity can be precisely controlled by determining the frequency of disulfide bridges. The presence of so much sulfur-containing cysteine is why heavily-keratinized tissues - like human hair - smell pungent when burned.
Sclerotins
Sclerotins are another widespread family of structural proteins, found primarily in arthropods but also some annelids. Sclerotins are a much broader family of proteins than keratins, and do not have a particular identifiable geometric structure, unlike the α-helixes and β-pleated sheets of keratins. They are also not bonded by disulfide bridges - instead, they are cross-linked by a process, called sclerotization, similar to what is done to tan animal hide into leather.
Sclerotin proteins contain large numbers of free amine and thiol groups, which tend towards oxidative reactions with other molecules. In the process of sclerotization, quinones are enzymatically introduced to tissue containing large numbers of sclerotins, typically by enzymatic action on dopamine-derivatives such as N-acetyldopamine. These quinones then react, forming strong covalent bonds, with the amine and thiol groups on the sclerotins, cross-linking them together into an exceptionally tough, hard, and rigid material. Like keratins, the exact degree of hardness and rigidity can be controlled, this time via the amount of quinones introduced into the tissue.
Sclerotization is effectively non-reversible - cross-linked sclerotins being extremely hard to break apart - and thus organisms must shed sclerotinized parts if they need to change them, hence the necessity of molting in arthropods.
Some annelids, such as the intimidatingly-named bloodworm, have special sclerotins which bind metal ions, such as copper and iron, prior to undergoing sclerotization. This results in unique metal-composite structures which are exceptionally hard and strong.[1]
Other cross-linking proteins
Other cross-linking proteins are found in other clades, though none as archetypical and widespread as keratins and sclerotins.
For example, cephalopod mollusks possess their own particular cross-linking proteins that add hardness and rigidity to their beaks, operating in a two-tiered system - they are, unfortunately, absent a catchy name like keratins and sclerotins, and are instead simply referred to as chitin-binding beak proteins (CBPs) and histidine-rich beak proteins (HBPs). CBPs simply bind to multiple chitin chains at both ends, forming cross-links between them. HBPs add a second tier to this matrix, and bond to both CBPs and each other, forming an additional set of cross-links to add even more strength, hardness, and rigidity to the result.[2]
Alien organisms may be expected to exhibit yet more variations on the common motif of proteins cross-linked together, unseen on Earth - instead of directly using one found on Earth, it may be better to simply specify a, say, "keratin-like protein"!
Biominerals
While all the structural materials discussed previously - and those which will be discussed after - are polymers, mineral crystals also play a major role in structural biological materials. All biominerals excel at adding hardness, strength, and rigidity to biological materials - for obvious reasons, heavily-biomineralized tissue cannot be flexible; although semi-flexible, partially-biomineralized tissue, such as cartilage in vertebrates, is observed in nature.
In addition to performing structural functions, biomineralized tissue also often functions as a reservoir of minerals - such as calcium, phosphate, and sulfate - that are essential for biological function. In fact, many heavily-biomineralized structures are believed to be evolutionarily derived from simple mineral reservoirs - it is believed that the spine in vertebrates originates from the use of the primitive notochord for calcium storage, for instance.
Most biominerals are salts, and they are typically classified first by their anion, then their cation.
Silicates

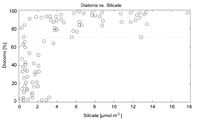
Silicates, otherwise known as common glasses (literally, like the kind in your window), are perhaps the most common and widespread class of structural biominerals on Earth, having independently evolved countless times throughout the tree of life. This may seem surprising, given that you probably know of very few living things that are made out of glass - and that's because almost all organisms that use silica as a structural biomineral are unicellular. Of the multicellular, macroscopic clades that use silica as a structural material, there is only one that uses it as primary structural material - the Hexactinellid sponges, otherwise known as "glass sponges." However, some plant clades, such as horsetails, do make use of it as a minor component.
Silicates are also one of the few biominerals that are not typically found in the form of a salt - instead being typically formed of silica (SiO₂) in its various crystalline forms. Silica is virtually impossible for any living organism - including plausible alien organisms - to metabolize for any purpose, as it requires extreme amounts of energy to break down into silicon and oxygen, and doesn't react with much of anything - as a result, unlike many other biominerals, it is solely a structural material. On the other hand, this strength and non-reactivity make it exceptional for that very purpose!
Silicate biomineralization is ususally performed by precipitating silic acid, dissolved in water, onto a matrix of structural filaments. This not only results in a very strong structural material, it is also, energetically, exceptionally cheap - organisms that use silica as a structural material spend only a fraction of the energy building such structures that other structural materials require.
This, however, means that building biological structures out of silica is strictly limited by the bioavailability of silicic acid - and silica does not, typically, readily dissolve in water, making this quantity limiting indeed. In fact, fierce competition over silicic acid is why, today, Heaxactinellid sponges are limited to deep-water environments, even though fossil evidence indicates they were once found in shallow waters - the evolutionary ascendancy of the unicellular diatoms, which build their frustules out of silica, depleted the surface waters of silicic acid, driving other shallow water organisms that utilized it to extinction. So, if you wish to have much larger, more complex, more numerous organisms use silicate biominerals, something must lead to a greater bioavailability of silic acid in the environment - for example, silica is significantly more soluble in water that contains a significant percentage of ammonia!
Carbonates
Carbonates are another exceptionally common class of biominerals, defined by the presence of the carbonate anion (CO₃⁻²). Due to the ubuiquity of both carbon and oxygen in biology - and in the environment in general - it is readily provided as an anionic component of biominerals.
Though sodium and potassium cations are exceptionally common in biology, sodium carbonate and potassium carbonate are both highly soluble in water; this makes them inadvisable as structural materials, as they will simply dissolve if they get wet (and water-born organisms are, essentially, always wet)!
Calcium carbonate
Typically, the cation in carbonate biominerals is Calcium (due to both its relative commonality and biological essentiality for most Earth life), and it is typically in the form of calcite (as in foraminifera, coccolithophores, and echinoderms) or aragonite (as in mollusk shells and coral skeletons), but calcium carbonate has also been observed in life in both vaterite and amorphous forms as well. Notably, presence of magnesium inhibits the formation of calcite - if significant magnesium is present in biomineral, it will almost certainly be in the aragonite form.
Calcium carbonates are highly soluble in acidic solutions - so on worlds where the waters are relatively acidic, calcium carbonate biominerals can not be expected to be used!
-
Calcite tests of foraminifers.
-
Cutaway of a nautilus shell, made of nacre - tiny aragonite plates layered on top of each other.
Magnesium carbonate
Magnesium is almost as widely used in biology as calcium, and is about half as common as calcium in Earth's crust and oceans. In fact, calcium carbonates frequently contain some amount of magnesium as the cation instead.
Magnesium carbonate - that is, where magnesium is by far the most predominant cation - will almost always be in the form of magnesite. If there are roughly 3 magnesium cations to each calcium cation, it will form huntite. If, instead, the mineral is composed of roughly equal portions of calcium and magnesium, it will form dolomite instead. Notably, higher-magnesium content carbonates are significantly less soluble in acidic solutions than calcium carbonate, meaning magnesite, huntite, and dolomite biominerals may be preferred on worlds with acidic oceans!
Dolomite and magnesite are also harder than aragonite and calcite, making them potentially stronger as structural components than the calcium carbonates. Huntite is, unfortunately, quite weak, making it generally inferior for structural purposes to both the calcium carbonates and the other magnesium carbonates. That huntite is in the middle of the transition between dolomite and magnesite means it will likely be somewhat more difficult to evolve near-pure magnesite biominerals for structural purposes than dolomite.
Manganese carbonate
Manganese is yet another biologically essential cation, though it is a quarter as common as calcium and half as common as magnesium. It is especially common on and around hydrothermal vents. Like with magnesium, it often accompanies calcium carbonates (and vice versa) in nature. Manganese carbonate is almost always found in the form of rhodochrosite - which, interestingly, has an intrinsic deep-red to pink color, with higher calcium content leading to lighter shades. Rhodochrosite is not observed as a structural biological material in nature, to my knowledge, but it is entirely plausible that alien organisms might use it as such.
Rhodochrosite is of comparable hardness to dolomite and magnesite, but also possesses the interesting property of perfect cleavage - that is, when rhodochrosite is cleaved along one of its cleavage planes, it leaves a perfectly smooth cut. This makes it resistant to fracture - it is almost impossible to fracture, rather than cut, in combination with its relative softness, in fact - and exceptionally difficult to cut along an axis that is not a cleavage plane. Since organisms can control the orientation of individual crystals when forming biominerals, this allows the possibility of composites with very particular rhodochrosite crystal orientation that are much tougher and stronger than expected.
Rhodochrosite is also denser and heavier than calcium and magnesium carbonates, with a specific gravity of 3.7 compared to the ~3.0 possessed by the others. This may be an advantage or disadvantage, depending on the application.
Manganese carbonate is also found in the form of Kutnohorite, when found with calcium, manganese, and iron. It is typically a pale, opaque pink. It has roughly the same hardness as rhodochrosite, but a lower specific gravity of 3.12.
Iron, copper, and zinc carbonates
Iron, copper, and zinc, while not as common as either calcium or magnesium, are biologically essential metals; and, indeed, they do appear as components of other biominerals, though I am unaware of any specific examples of iron carbonate or copper carbonate biominerals, specifically.
Iron carbonate is typically in the form of siderite, though compounded with calcium and some amount of manganese it instead forms ankerite. Both are of comparable hardness to dolomite, magnesite, and rhodochrosite; siderite has a hardness ranging from 3.75 to 4.25, while ankerite has a hardness ranging from 3.5 to 4. Siderite is substantially heavier than even rhodochrosite, with a specific gravity of 3.96, though ankerite has roughly the same specific gravity as calcium and magnesite carbonates.
Copper carbonates are only found in the carbonate hydroxide forms, specifically azurite and malachite. The difference is the ratio of hydro carbonate to hydroxide; azurite has a roughly 1:1 ratio, while malachite has a ratio of roughly 1 carbonate:2 hydroxide. Azurite is intensely blue, and malachite is intensely green; azurite's hardness ranges from 3.5 to 4, while malachite's ranges from 3.5 to 4.5. Azurite will slowly weather to malachite, when exposed to atmosphere.
Zinc carbonate is found in the form of smithsonite when it is the sole cation, or in the form of minrecordite when compounded with equal amounts of calcium. With hydroxides, it can be found in the form of hydrozincite; with hydroxides and copper, it can be found in the forms of rosasite and aurichalcite. Smithsonite is quite hard, with a hardness of about 4.5, and a very high specific gravity of approximately 4.5. Minrecordite, technically a variety of dolomite, has roughly the same properties as dolomite. Hydrozincite is, unfortunately, not very hard, and thus not particularly suitable for structural use, as is auricalchite; rosasite, however, has a high hardness of 4 and a high specific gravity of 4-4.2.
Phosphates
Phosphates, while not as common as carbonates (either in biominerals or in life in general), possess two properties which make them especially desirable as biominerals.
First, phosphate is itself an absolutely essential nutrient for all life on earth, and the same can be expected to be so as well for extraterrestrial organisms which have even remotely similar biochemistry to earth organisms, due to being the primary source of the element phosphorous. Therefore, phosphate biominerals serve not only as a reservoir of whatever cation the phosphate is paired with, but of phosphate as well.
Second, phosphate minerals are generally harder - and thus make for stronger composites - than carbonates, typically ranging from around 5 to 6 in hardness, compared to carbonates' typical 3 to 4, while being no heavier.
As with carbonates, sodium phosphate and potassium phosphate are too soluble in water to be used for structural biominerals - although, surprisingly, there are some sodium-containing phosphates that are insoluble enough to be viable.
Apatites - calcium phosphates
Calcium phosphates, otherwise known as apatites, are the most common phosphate biominerals here on Earth, again owing to calcium's biological essentiality and relative commonality. Apatites are always found with a third ion in addition to the calcium and apatite, and this can be one of three different ions - hydroxide (OH⁻), fluoride (F⁻), or chloride (Cl⁻). All apatites have a hardness of 5 (for which they are the defining mineral) and a low specific gravity of 3.16-3.22.
Hydroxyapatite, an apatite with hydroxide (OH⁻), is the most common biomineral apatite on Earth - and also likely to be so on alien worlds, given the relative rarity of fluorine and chlorine. It is the primary mineral constituent of vertebrate bone (though some carbonates are also included), and is also found in a the clubbing appendages of the peacock mantis shrimp.
Fluorapatite, a less biologically common apatite with the fluoride ion (F⁻), is actually quite commonly found in Earth life - particularly because fluoride, in solution, will readily replace the hydroxide in hydroxyapatite, transforming it into fluorapatite. That fluorapatite is slightly stronger and more chemically resistant than hydroxyapatite is the reason why, here on Earth, water in developed countries has fluoride added - the replacement of hydroxyapatite in your teeth with fluorapatite helps maintain dental health. Similarly, we can expect alien organisms to incorporate as much fluorapatite over other apatites as there is fluoride bioavailable to do so.
Chlorapatite, an even less biologically common apatite with the chloride ion (Cl⁻), is again, actually found in Earth life - like fluoride, it will replace hydroxide, but it will in turn be replaced by fluoride. It is mostly irrelevant on Earth, forming only in environments particularly deficient in fluorine, but if an alien world has significantly more chlorine than Earth, it may become a major biomineral.
Whitlockite - calcium magnesium/iron phosphate
Whitlockite is a distinct form of calcium phosphate that forms with the introduction of significant amounts of magnesium and iron, having the overall formula Ca₉(MgFe)(PO₄)₆PO₃OH, though the amounts of magnesium and iron can be varied more than this formula suggests. It is sometimes considered a subtype of apatite.
Whitlockite has a slightly lower specific gravity than (other) apatites, at 3.13, and is just as hard. Its crystalline structure is ditrigonal pyramidal, instead of the dipyramidal arrangement found in (true) apatites; it has a noticeably different texture as a result, and displays no cleavage. It is found in some parts of biological systems where one would ordinarily find hydroxyapatite, on Earth organisms.
Manganese phosphates
Manganese, already mentioned to be a biologically important cation, forms a number of interesting - and potentially biologically useful - minerals with phosphate, typically including other cations, particularly calcium and iron, as well.
Graftonite - iron/manganese/calcium phosphate
Graftonite is an interesting phosphate mineral with a mixture of iron, manganese, and calcium, in variable proportions, as the cation, and no additional anions such as hydroxide, unlike in apatite. It has roughly the same hardness as apatite, with a rating of 5, and has a significantly higher specific gravity of approximately 3.7. Unlike the colorless-to-white apatites, due to the inclusion of manganese and iron, it has a red-brown to pink color, depending on the exact proportions of each cation.
Notable for its lack of hydroxides or fluorides, unlike most other phosphates.
Triplite and Triploidite - manganese/iron phosphate hydroxy/fluoride
Triplite and triploidite are two related phosphate minerals formed with a mixture of manganese and iron in variable proportions. It appears a pinkish red-brown in color, again varying somewhat towards red-brown or pink depending on the ratio of manganese to iron. The two names are essentially describing the two ends of a spectrum of minerals, with triplite referring to fluoride-heavy instances, while triploidite refers to hydroxide-heavy instances. The mineral's hardness is roughly equivalent to apatite, but varies depending on the ratio of hydroxide to fluoride, falling as low as 4.5 and reaching as high as 5.5.
Natrophilite - sodium manganese phosphate
Natrophilite is a particularly common phosphate mineral with both sodium and manganese as cations. It is a slightly pinkish yellow in color. It is slightly less hard than apatite, but still quite hard - rating between 4.5 and 5 on the Mohs scale - and has a slightly higher specific gravity of 3.41. Despite its slight weakness in comparison to apatite, it is still harder than all carbonate minerals, and is notable for being a sodium-containing mineral that is not particularly soluble in water - although it still readily dissolves in acidic solutions.
Alluaudite - alkaline manganese phosphate (+iron/magnesium)
Alluaudite is a somewhat complex mineral, relatively common - although mostly abiogenic - on Earth, with the complex chemical formula (Na,Ca)Mn²⁺(Fe³⁺,Mn²⁺,Fe²⁺,Mg)₂(PO₄)₃. In essence, this is a phosphate mineral, with a manganese cation, a sodium or calcium cation, and two cations of iron, (more) manganese, or magnesium. As befitting its variable composition, it has a variable coloration depending on composition, including dirty yellows, yellowish browns, and grayish greens.
This variable composition makes it surprisingly plausible as a biomineral - it could serve as a reservoir of a variety of different essential minerals for a living organism, in addition to serving a structural purpose.
It is notably harder than apatite, with a hardness of 5 to 5.5 on the Mohs scale. It has a somewhat greater specific gravity, from 3.4 to 3.5.
Sulfates
While much less common than silicates, carbonates, or phosphates, some organisms do use sulfate minerals for structural functions. Sulfate is, like phosphate, an essential biomineral for a variety of organism; though, unlike phosphates, sulfates are quite structurally weak, more comparable to carbonates. Perhaps surprisingly, this is one class of biomineral for which calcium is not the most common cation - calcium sulfate minerals are generally quite weak, and fairly soluble in water, making them poorly suited for structural purposes. Instead, the predominant cations that appear with sulfates are, surprisingly, the relatively rare barium and strontium, giving sulfate biominerals astoundingly high specific gravities compared to most other biominerals.
Baryte - barium sulfate
Barium sulfate is used as a reinforcing biomineral in a variety of clades across the tree of life on Earth, including some close relatives of the plants (embryophtes), such as the Charophyceaen and Zygnemophyte algaes. It is relatively colorless, and has a Mohs scale hardness of 3-3.5. It has a specific gravity of 4.3-5 - bare in mind, no non-sulfate biomineral we have described thus far has had a specific gravity above 4!
Rather than storing barium for later biological use, baryte, as a biomineral, tends to serve the purpose of keeping barium away - barium is toxic, and not particularly biologically useful, to nearly all living organisms. By joining it with sulfate, barium ions are precipitated out of water into baryte, where they both can no longer harm the organism and also can provide a convenient source of structural material.
Celestine - strontium sulfate
Celestine a prettily-named and quite pretty looking sulfate mineral, is, despite its seeming oddity, one that is actually found on Earth - the Acantarea clade of radiolarians make their hard skeletons out of celestine, the high specific gravity of which helps them cling to ocean floors, avoiding being carried away.
Celestine has a very pretty light blue color, a hardness of 3-3.5, and a very high specific gravity of approximately 3.95. It also happens to fluoresce yellow-to-light-blue in the UV range.
Metal Oxides and Sulfides
A few organisms, in need of exceptionally tough and hard biominerals, have evolved the use of metal oxides and sulfides for such a purpose. Unlike metal salts, they do not make particularly good reservoirs of essential metals for other biological purposes - they are quite strongly bound, and thus are, like silicates, almost solely used as structural materials. However, they do also potentially have one other, interesting use - many of these minerals (particularly magnetite and greigite) are ferromagnetic, and can function as a sort of biological compass, in special bodies called magnetosomes.
Magnetite and Goethite - iron oxides
Magnetite and Goethite are iron oxide minerals that are both found as biominerals here on Earth - magnetite in the teeth of chitons, and goethite in the teeth of limpets. It is no accident that both cases involve teeth - both clades scrape organic matter, such as algae and bacteria, from hard rocks and sediment, and need teeth that are exceptionally hard and resistant to wear.
Magnetite has an exceptionally high Mohs scale hardness of 5.5-6.5, and a very high specific gravity of approximately 5.18. Goethite has slightly less hardness, with 5-5.5, and a lower specific gravity of 3.3-4.3. Both are a glossy black color.
Pyrite and Greigite - iron sulfides
Pyrite and Greigite are iron sulfide minerals that both, again, are found as biominerals here on Earth - though, this time, in the defensive adaptions of gastropod molluscs living near hydrothermal vents, which reinforce their shells with these minerals.
Pyrite has an absolutely outstanding (for a biomineral) Mohs scale hardness of 6-6.5, and a high specific gravity of 4.95-5.10. It, infamously, looks quite similar to elemental gold - having the common name "fool's gold." Greigite has the more moderate hardness and specific gravity values of 4-4.5 and 4, respectively, and appears a blue-black color.
Polyesters
Given that polyesters are typically thought of as synthetic polymers, it may be surprising to hear that polyesters do, in fact, occur and play structural roles in Earth life - although, in only two known cases, both of which are relatively marginal. In particular, the cutin that makes up the waxy outer layer covering the leaves of plants; and a special polymer secreted by bees of the genus Colletes to protect their brood cells. Both cases are, unfortunately, poorly understood - but they show, without a doubt, that polyesters are viable as a structural biological material, and there's no reason why alien organisms might not make much wider use of them! Some bacteria also make use of polyhydroxylalkanoates as an energy and carbon storage mechanism, but it seems to serve no structural purpose.
Polyesters tend to be divided into two categories, based on the motifs present in their chemical structure, which are, incidentally, also the two classes of hydrocarbon: aliphatic polyesters, where carbon atoms form open chains; and aromatic polyesters, where the carbon atoms form closed, six-member rings. Aromatic polyesters tend to be much more crystalline in structure (and therefore much harder and more rigid), while also being more chemically and thermally stable. Most are, however, rather difficult for organisms to synthesize.
While the most well-known synthetic polyester - and an obvious thought when hearing that polyesters are viable structural biological materials - is polyethylene terephthalate, otherwise known as PET, the conditions under which this is produced are not suitable for biological processes. There are, however, a number of other interesting polyesters to look at.
Polyesters from hydroxy acids
All polyesters that appear in nature here on Earth - although, this is a rather limited selection, given their relative rarity - are, it seems, derived from hydroxy acids (that is, carboxylic acids with at least one hydroxyl group), particularly short-chain fatty acids. The exact chemical mechanisms by which biochemical processes produce these polyesters is generally poorly understood, although polyhydroxylalkanoates are known to be produced via action of an enzyme on the hydroxy acids.
Cutin
Cutin, along with the related polymer suberin, is what provides plants with the tough, waxy outer coating of their leaves, assisting them in retaining their structure and making them hydrophobic so that rain slides off their surfaces. While it is not very well understood, it is thought to be a branched polymer of C16 and C18 hydroxy fatty acids, such as hydroxy palmitic acid. These very-long-chain acids, and its branching structure, result in an amorphous, soft, waxy structure, that is nonetheless fairly tough, completely insoluble in water, and highly hydrophobic.
Polyhydroxylalkanoates
Polyhydroxylalkanoates, such as polyhydroxylbutyrate, are another polyester that appears in nature - albeit not for structural purposes. It is used by a number of species of bacteria for energy and carbon storage. Nevertheless, it still provides an option to us for a structural biopolymer, as, on Earth, polyhydroxylalkanoates are routinely harvested from the bacteria that produce them and used to make strong plastics, particularly for biomedical use (as they are non-toxic).
As a structural material, polyhydroxylalkanoates are generally quite ductile, and may or may not be quite elastic depending on exact composition. They are tough and strong, with similar tensile strength to polypropylene. They are generally permeable to oxygen, but not to water. Compared to other polyester plastics, they also tend to have quite good UV resistance.
Phenolic acid polyesters
Phenolic acids - that is, a phenol with a carboxyl group, such as salicylic acid[3] - which are widely found in nature, have been demonstrated to be capable of forming polyesters under a variety of conditions. In the case that these are homogenous, linear polyesters (i.e. have no chain branches), their properties should generally be similar to other aromatic polyesters - that is, highly crystalline, and therefore hard and rigid.
More complex polyphenolic acids may instead more closely resemble polyphenols - indeed, phenolic-acid polyesters are both polyphenols and polyesters!
Polyesters from lactones
Lactones can be readily converted into polyesters via a variety of catalytic ring-opening polymerization reactions. While there are, as far as I am aware, no natural polyesters that are derived this way, lactones do occur in nature, and catalytic processes are well-suited for biochemistry.
Polylactic acid
Polylactic acid, most often known by its acronym "PLA", is a plastic most known, here on Earth, for its use as a 3D-printing filament. While it is not found in nature, it is catalytically synthesized from lactide (the lactone of lactic acid), which very much is, meaning alien organisms that synthesize it are highly plausible.
Unlike most other aliphatic polyesters, PLA ranges from semi-crystalline to highly crystalline in structure depending on exact composition, and is thus quite hard and rigid - it is somewhere in between polystyrene and PET in mechanical properties.
PLA is also sensitive to UV degradation, but this simply breaks off monomers, which could be biologically recuperated and the PLA repaired.
Polycaprolactone
WIP - needs more research
Polybutyrolactone
WIP - needs more research





