Structural Biological Materials
Real organisms use a wide variety of structural materials to give toughness, strength, rigidity, and other desirable material properties to their bodies, both on the cellular (as in cell walls) and tissue (as in bones or exoskeletons) level. Moreover, these materials are rarely homogenous - biological structural materials are almost always composites of more than one material, becoming something greater than the sum of its parts.
Rather than simply take the approach of listing options, this page will aim to explore the general categories of structural biological materials - both real and theoretical - and explain how their particular properties play into how they're used in living creatures.
Polysaccharides (cellulose, chitin, algin, etc.)
Polysaccharides are perhaps the most widespread - and simplest to evolve - structural materials we find in living creatures. Saccharides, especially glucose, are universally used as energy storage in Earth life, and it's likely that alien life - at least, that which shares our basic biochemistry - will do the same. It therefore isn't much of a step to derive your structural materials from the stuff you already use for energy storage!
Most polysaccharides are hydrophobic - even though their component monomers readily dissolve in water.
Cellulose, and other simple linear polysaccharides
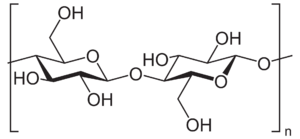
Cellulose is the simplest and most widespread polysaccharide that sees structural use on Earth, and is likely to be so on alien worlds as well. It's a simple linear chain of β(1→4) linked units of d-glucose - the same molecule nearly all life on Earth uses to store energy. Aliens might use ʟ-glucose instead, but this should have identical mechanical properties.
Contrary to popular belief, cellulose is actually quite flexible, when alone. The rigidity of plant cells - famously with cell walls made out of cellulose - is provided by other materials bonded with cellulose, such as lignin, along with the hydrostatic pressure provided by the water within the cell.
A number of Earth organisms are demonstrated to use mannose, or other simple sugars, in the place of glucose, forming structural polysaccharides such as mannan. Their properties are similar to cellulose - though, how they may bond to other polymers might differ, as we'll discuss later with glucomannans.
Some other organisms, such as oomycetes, use other β-glucans - that is, linear chains of glucose - which have different linkages (such as the β(1→3) linkages of chrysolaminarin) as structural materials. In bulk, their mechanical properties are fairly similar.
Chitin, and other poly-amino-sugars
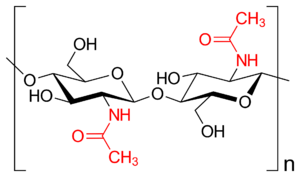
Chitin is the second most widespread structural polysaccharide on Earth, and for good reason. Like cellulose, it is a linear chain of single monomers. Rather than being made of simple glucose, however, it is composed of N-acetylglucosamine - an amino-sugar of glucose where one of the hydroxyl groups is replaced by an acetylamino moiety. This acetylamino moiety forms stronger hydrogen bonds with adjacent polymers than the hydroxyl group would, rendering chitin much stronger and slightly more rigid than cellulose - though, it still remains plenty flexible, without other components to rigidify it.
N-acetylmannosamine and N-acetylgalactosamine, derivatives of mannose and galactose, respectively, also exist in nature - to my knowledge, they are not used to form polymers like chitin, but an alien organism may do so!
Algin, and other polyuronic acids
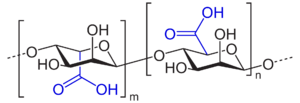
Algin, or alginic acid, a polyuronic acid, is a primary structural component of the cell walls of brown algae, such as kelp. It is a polymer of two uronic acids in alternating sequence - β-D-mannuronate and α-L-guluronate. Uronic acids are derivatives of derivatives of saccharides where the hydroxyl group opposite the carbonyl has been oxidized to a carboxyl group, making them a kind of carboxylic acid. This makes polyuronic acids acidic (as implied by the name), which in turn makes them hydrophilic and water-soluble. Since these are long polymers, and typically cross-linked, when used as structural materials, however, they do not exactly dissolve - instead, infiltration of water into the polyuronic acid matrix forms a sort of hydrogel. This is what gives brown algae their soft, gel-like, yet still quite tough structure.
Disaccharide polysaccharides
As demonstrated by algin, above, structural polysaccharides, rather than consisting of a homogenous chain of a single monosaccharide molecule, are instead often composed of two distinct monosaccharides in alternating sequence. This allows the copolymer to have some of the properties offered by both monosaccharides.
A prevalent example in nature is the polysaccharide component of the peptidoglycan in bacterial cell walls, here on Earth - it is an alternating copolymer of N-acetylglucosamine (as found in chitin) and N-acetylmuramic acid, a complex monosaccharide where a lactic acid group has replaced yet another hydroxyl group on N-acetylglucosamine. This lactic acid group is used to provide a strong binding site for the oligopeptide component of the peptidoglycan, which crosslinks the polysaccharide chains.
There are a number of such other examples of alternating polysaccharide copolymers in nature, and there are likely to be yet more combinations in alien organisms!
Branching polysaccharides
As seen above, almost all polysaccharides used for structural purposes in living organisms are linear chains, though branching polysaccharides are commonly used for energy storage. However, some branching polysaccharides do, in fact, see structural use - such as the glucomannans found in some yeasts and plants. Glucomannans consist of a linear chain of mannose units (called a mannan), with short glucose branches. Unlike linear polysaccharides, branched polysaccharides resist forming neat, crystalline structures (as the branches interfere with hydrogen-bond formation between polymers), and thus are able to slide past each other, forming a rubbery, hydrophilic solid.
Polyphenols (lignin, tannin, etc.)
Polyphenols, such as lignin and tannin, are a broad category of biopolymer found almost exclusively in plants on Earth, consisting of many phenol units oxidatively coupled together. Unlike other common structural biopolymers, polyphenols are typically non-linear - instead forming complex 3-dimensional structures. They are also often highly heterogenous, made of many different monomers. This makes it difficult to talk about sub-types of polyphenol, as we have done for polysaccharides - instead, this section will talk about the properties different polyphenols often possess.
Color
Polyphenols' complex 3d structures leads to a variety of interactions with light waves that result in them being typically opaque and strongly colored. While most polyphenols are dark brown - lignin being responsible for the dark brown color of most tree bark - some polyphenols have brilliant colors, such as the anthocyanidins that provide flower petals with vivid reds, purples, and blues.
While structural polyphenols are typically quite separate from pigment polyphenols, an organism that is able to synthesize one is likely capable of synthesizing the other!
Reactivity
Whereas most structural biopolymers are only weakly reactive, polyphenols are quite reactive, specifically to oxidation. This might sound like a vulnerability for a structural material, but polyphenols' reactivity is usually localized to a few specific sites on the molecule. This makes them very good at binding to other structural polymers, like polysaccharides and proteins, at multiple sites, via oxidative coupling. Other structural biopolymers bound by polyphenols in this way tend to become exceptionally hard and rigid - like tree bark.
Resistance to degradation
Some - not all - polyphenols, such as lignins, are exceptionally resistant to bio-degradation, making them very difficult for other living things to break down and digest - and this extends to the other polymers they bind to. This makes them very good at protecting from penetration by parasites or pathogens, and limits the possible predators of organisms that use polyphenol cross-linking in their structures.
Cross-linking proteins (keratins, sclerotins, etc.)
Proteins - perhaps unsurprisingly, given their ubiquity - are common components of structural biological materials, specifically in the form of cross-linking proteins like keratins or sclerotins, which bond to each other at many different sites, allowing for not only toughness and rigidity but a tunable degree of both - via controlling the frequency of cross-linkages, materials can smoothly vary in rigidity and hardness, often forming distinct gradients in biological structures (such as arthropod cuticle becoming more flexible around the joints).
All cross-linking structural proteins tend to be brown to black in color.
Keratins
Keratins are the primary family of structural proteins found in vertebrate integument - composing hair, nails, feathers, scales, etc, while also reinforcing the epithelial tissue (the outermost layer of the organs and blood vessels). There are two primary categories of keratin - α-keratins, which are found in all vertebrates, and β-keratins, which are found only in sauropsids. The primary difference between these is that α-keratins form double-helix filaments and are more flexible, whereas β-keratins form β-pleated sheets and are significantly harder and more rigid.
Both types of keratin crosslink by means of disulfide bridges - they contain large amounts of the amino-acid cysteine, which bonds to cysteines on adjacent keratin polymers via the sulfur. These bonds are very strong and rigid, and, as mentioned before, the precise degree of hardness and rigidity can be precisely controlled by determining the frequency of disulfide bridges. The presence of so much sulfur-containing cysteine is why heavily-keratinized tissues - like human hair - smell pungent when burned.
Sclerotins
Sclerotins are another widespread family of structural proteins, found primarily in arthropods but also some annelids. Sclerotins are a much broader family of proteins than keratins, and do not have a particular identifiable geometric structure, unlike the α-helixes and β-pleated sheets of keratins. They are also not bonded by disulfide bridges - instead, they are cross-linked by a process, called sclerotization, similar to what is done to tan animal hide into leather.
Sclerotin proteins contain large numbers of free amine and thiol groups, which tend towards oxidative reactions with other molecules. In the process of sclerotization, quinones are enzymatically introduced to tissue containing large numbers of sclerotins, typically by enzymatic action on dopamine-derivatives such as N-acetyldopamine. These quinones then react, forming strong covalent bonds, with the amine and thiol groups on the sclerotins, cross-linking them together into an exceptionally tough, hard, and rigid material. Like keratins, the exact degree of hardness and rigidity can be controlled, this time via the amount of quinones introduced into the tissue.
Sclerotization is effectively non-reversible - cross-linked sclerotins being extremely hard to break apart - and thus organisms must shed sclerotinized parts if they need to change them, hence the necessity of molting in arthropods.
Some annelids, such as the intimidatingly-named bloodworm, have special sclerotins which bind metal ions, such as copper and iron, prior to undergoing sclerotization. This results in unique metal-composite structures which are exceptionally hard and strong[1].
