Nuclear radiation
Atomic nuclei can store far more energy than chemical bonds. When a high energy nucleus becomes a low energy nucleus, it needs to shed that excess energy somehow. It does this by emitting particles or waves that carry away most of that energy. These particles or waves move away from the nuclear material in all directions – they radiate away, and hence this phenomenon is called nuclear radiation.
A similar process can occur with sub-atomic particles. Some varieties of these can react to distribute their energy among various other kinds of particles or waves that radiate away. Because these particles or waves are also highly energetic, they behave in a very similar fashion to nuclear radiation and can largely be handled in the same way.
(By quantum mechanics, all particles are also waves and all waves can be represented as particles. Consequently, from here on out, we'll just use "particle" to refer to both particle and wave radiation behavior.)
Processes
Radioactivity
Nuclei with stored energy can be unstable. Given time, they can spontaneously decay, releasing their energy as radiation[1]. These unstable nuclei are called radioactive, and the process of their decay is radioactivity. Note that, despite having similar sounding names, radioactivity is separate from radiation – if you protect yourself from one, you are not necessarily protecting yourself from the other. Penetrating radiation emerging from radioactivity can get through barriers that will keep the radioactive material out, and all the shielding in the world will not help you if the radioactive material can get to your side of the shielding.
Radioactive material where you do not want it is called radioactive contamination.
The original radioactive nucleus is called the parent nucleus, and the nucleus it decays into is called the daughter nucleus.
Sub-atomic particles behave in the same way, with unstable particles decaying to more stable particles by emitting radiation. They are also radioactive. However, sub-atomic radioactivity tends to occur at a much faster rate than nuclear radioactivity, such that it is essentially instant from a human time scale.
Radiactive decay
In any given span of time, a given fraction of the radioactive material in any sample (as measured from that present at the start of that span of time) will decay[1]. It is convenient to find the time it takes for exactly half of the radioactive material to decay, this is called the half-life and is commonly denoted with t½ in equations. In another half-life after the first, half of the remaining material will decay and thus you will be left with one-half of one-half of the original sample, or one quarter of the original amount. Similarly, after three half-lives, you will have 1/8th of the original material; after four half-lives, 1/16th of the original material, and so on. In general, after n half-lives, 1/2n of the original sample will still be present. After many half-lives, a sample will have decayed away to the point where it is negligible.
When doing calculations, the half-life can be inconvenient to use. It is more convenient to define a characteristic decay time τ which is related to the half-life by
τ = t½ / ln[2].
After any arbitrary amount of time t when starting with an amount N0 of radioactive material, the amount of remaining material will be
N(t) = N0 exp[-t/τ].
If N is measured in number of atoms, the rate at which the decays occur is
A = N / τ.
This rate is called the activity of the sample. Note that while a long half-life means that you need to deal with the radioactivity for a long time, the overall activity will be low. Meanwhile, an isotope with a short half life may go away quickly but will have a high activity during that time.
It is also occasionally useful to note that τ is the average life span of any given radioactive particle.
Decay chains
You can often find yourself in a situation where a parent nucleus decays into a daughter which is itself unstable. You can get a whole sequence of decays between unstable nuclei before you settle down into a stable state. This is called a decay chain[1].
It is important to keep decay chains in mind; just because a parent has gone through so many half-lives that essentially none is remaining it does not necessarily mean that all the radioactivity is gone if there are daughter products with longer half-lives that were produced by the sample.
Further, just because the parent to daughter decay might produce a relatively benign form of radiation does not mean that you don't get nastier radiation from decays further down the decay chain.
An example of a decay chain from one of the most common naturally occurring radioactive isotopes on our planet is[2]
238U →
234Th →
234Pa →
234U →
230Th →
226Ra →
222Rn →
218Po →
214Pb →
214Bi →
214Po →
210Pb →
210Bi →
210Po →
206Pb
The final daughter product, 206Pb is stable.
If you have a chain of daughters with half-lives that are much shorter than that of the parent, any initial excess of the daughter products in your sample will quickly decay away on the scale of a parent half-life to the point where the only daughter products present are those produced by the parent decay. Any initial deficit will build up over a similar time scale until the rate of production of the daughter products equals their rate of decay – and when you follow the chain back to the parent, the rate of production is the same as the rate of parental decay. Thus, all the daughter products in such a chain will have the same activity as the parent, until you reach a daughter product with a longer half-life. This is called secular equilibrium.
Fission


When a very heavy nucleus is disturbed, it can deform and elongate. Because the two ends of the elongated nucleus are both very highly positively charged, they strongly electrically repel each other. Normally, this is countered by the strong nuclear force gluing the nuclear particles together, but if the nucleus elongates it gives a lower cross sectional area for the nuclear force to stick the nucleus together. This leads to a runaway process, with the two ends pushing each other apart as the center thins out, forms a neck, and finally snaps. Which leaves two now separate new nuclei where once there was one; usually with one nucleus a bit heavier than the other. This process is called fission[1].
The fission process typically flings off some excess neutrons that are not needed in either of the two new nuclei. The rapidly changing charge configuration also usually throws off several gamma rays. In addition, the two halves of the original nucleus are thrown apart violently by their mutual electric repulsion, giving two heavy ions to go careening through the material. The exact energy and particle distribution will vary from fission to fission, but "typical" values might be
- Ions: 165 MeV distributed between the two ions, of which the light ion has about 2/3 the energy of the heavy ion.
- Neutrons: 5 MeV; split among an average of about 2.5 neutrons with approximately 2 MeV each.
- Prompt gamma rays: 8 MeV, typically about 0.5 MeV each
- Delayed gamma rays from subsequent beta decay of the fission fragments: 7 MeV, typically 1-2 MeV each
- Delayed beta particles from subsequent beta decay of the fission fragments: 7 MeV, typically 1-2 MeV each
- Delayed neutrinos from subsequent beta decay of the fission fragments: 12 MeV (one for each beta particle produced)
For some heavy nuclei, the energy of neutron capture is enough to kick-start this process and thus will readily fission in the presence of thermal neutrons. These isotopes are called fissile. Other isotopes can fission but which usually do not do so unless hit hard enough by a fast neutron or high energy gamma. Some isotopes can even undergo fission on their own, as a process of radioactive decay; such behavior is called spontaneous fission. Any isotope that can undergo fission, fissile or not, is called fissionable.
Fusion
Nucleons – protons and neutrons – are sticky, and when they touch they tend to bind together via the strong nuclear force. This is opposed by the electric force, which repels the protons from each other. For light nuclei, the glue of the strong nuclear force overcomes the mutual repulsion of electromagnetism, and these light nuclei can release energy by sticking together. This process is called fusion[1]. The energy that is produced in the process must be emitted in another form, and this process is nuclear radiation.
For all viable artificial forms of fusion, the emitted radiation is in the form of nuclear particles that were already present in the two fusing nuclei. The new clump of fused protons and neutrons must throw off some of their number in order to shed the excess energy. The most favorable fusion reaction – the fusion of deuterium and tritium – always de-excites by throwing off a very energetic neutron with 14.1 MeV and kicking the remaining alpha particle in the other direction with 3.5 MeV of energy. Consequently, most of the energy comes off in the form of fast neutron radiation. Another common reaction is the fusion of deuterium with deuterium, which becomes a 2.5 MeV neutron and 0.8 MeV helion ion half the time and a 3 MeV proton and a 1 MeV triton half the time (the trition will then nearly always go on to react with the deuterium fuel present to give you an additional deuterium-tritium fusion). Deuterium-deuterium fusion is an order of magnitude harder to ignite than deuterium-tritium, but it is still easier than most other fusion reactions.
Because neutron radiation is annoyingly penetrating, and tends to mess with the materials that make your reactor, there is considerable interest in reactions that produce fewer neutrons. The easiest of these to ignite – nearly as easy as deuterium-deuterium (which is still very hard) – is deuterium-helium 3 fusion. This produces a 14.7 MeV proton and a 3.6 MeV alpha particle. Both of these are charged and so they can be easily stopped in matter or confined with magnetic fields. However, the reacting plasma is so hot that the electrons whizzing around the plasma produce copious bremsstrahlung x-ray radiation, which is uncharged. In addition, some deuterium-deuterium side reactions produce neutrons, so you're not entirely neutron free.
Proton-boron 11 fusion is often hailed as a nearly neutron-free reaction, as it always results in three alpha particles and a gamma ray. However, it is so hard to get going that it appears to always emit more energy in bremsstrahlung than it gains by fusion, which handily stops the reaction from happening.
The fusion that occurs inside stars is far too slow and low yield to ever be viable for an artificial fusion reactor. Although there are many such reactions that can occur, they all emit radiation. For example, that which predominates in our own sun, the proton-proton chain, emits nuclear gamma rays and positrons which produce annihilation gamma rays.
Consequently, any form of nuclear fusion will be an intense source of nuclear radiation.
Particle beams
Particle Accelerators produce beams that consist of ionizing radiation, and drive processes and interactions that scatter this radiation out of the beam and produce more and different kinds of radiation.
Kinds of radiation
Alpha and Ions
An atom or nucleus set moving through a material faster than about 1% of the speed of light will be stripped of its electrons, forming a bare ion. This will leave extensive ionization tracks. Ions are short ranged in matter, as the massive particles are relatively slow-moving and highly charged, and thus leave tracks of very high levels of ionization that quickly sap off the kinetic energy and bring the particle to a stop. After the ion is slowed to less than about 1% of light speed, it causes less ionization but can cause extensive displacement of atoms near the end of its track. Ions can come from fission, from particle accelerators, and from radioactive decay.
Alpha radiation is what you get when an energetic nucleus sheds its energy by throwing off an energetic 4He nucleus (two protons and two neutrons all stuck together, often called an alpha particle when it is emitted as radiation). 4He is very stable and tightly bound, thus favoring its emission over other nuclear particles such as protons[1]. This radiation is one form of ion radiation, but it is so common that it has its own name. Alpha particles travel a few centimeters through air, and are stopped by a sheet of paper or the outer (dead) layer of your skin. Alpha radiation is mainly dangerous when it happens inside your body (see the sections on radioactivity and radioactive contamination, which despite the similar name are separate concepts from radiation). The high concentration of ionization from the alpha particles can cause serious biological effects on living cells.
In addition to alpha particles, there are other specific ions that have special names:
- Protons are the lightest ions, and are the nuclei of almost all hydrogen atoms (1H).
- Deuterons are the nuclei of deuterium, a rare form of hydrogen (2H). Deuterons are a proton stuck to a neutron.
- Tritons are the nuclei of tritium, a radioactive, man-made form of hydrogen (3H) with a 12.3 year half-life. Tritons are a proton stuck to two neutrons.
- Helions are the nuclei of the 3He isotope. They consist of two protons stuck to one neutron.
Beta
Beta- radiation happens when a nucleus turns a neutron into a proton via the weak nuclear force; a process that emits an electron (the beta particle) and an electron anti-neutrino as radiation[1]. The resulting proton stays inside the nucleus, but the electron and anti-neutrino escape. Neutrinos almost never interact with matter, so it can be ignored from here on out. However, the electron is charged so it will leave behind an ionization track. Because the electron moves much faster than an alpha particle (nearly the speed of light), it leaves a much sparser ionization track and thus has a significantly longer range through matter. Electrons from nuclear radiation can travel on the order of a meter through air, and can reach the living tissues of the skin, causing sunburn-like radiation burns. They can be stopped by a thin sheet of aluminum foil or a stack of several sheets of paper. If beta emission occurs inside an organism (likely because of internal radioactive contamination), it can cause whole body radiation exposure although the sparse ionization tracks are less damaging than those from alpha radiation of equal energy.
Beta+ radiation is a rarer process that happens when a proton turns into a neutron and emits an electron neutrino and a positron (the beta particle) as radiation. Again, the resulting neutron stays in the nucleus, but the neutrino and positron escape. The positron behaves almost the same way as the electron from beta- decay, except that at the end of the radiation track when it is slowed to a near stop it will encounter an electron and annihilate. This produces a pair of gamma rays (see below) of a very characteristic energy that can be used to identify beta+ activity.
Electron capture is a process that competes with beta+ activity. In this case, a proton turns into a neutron not by emitting a positron but by capturing one of the electrons orbiting the nucleus. This produces only the electron neutrino as radiation, although there may be some shake-up of the electron shell leading to x-rays and auger electrons.
Gamma
When a nucleus is in an energetic state that has the same number of protons and neutrons as a nucleus in a less energetic state, it can transition to the lower energy nucleus by emitting a gamma ray to conserve energy. A gamma ray is a quanta of oscillation of electromagnetic radiation with very high frequency. Essentially, as the electric charges in the nucleus re-arrange themselves into a more stable state with a rapid collapse of their configuration, some of the electric field they created is left behind in the process. This left-over field creates an oscillating electromagnetic wave that is called the gamma ray[1].
Nuclei with the same number of protons and neutrons but different internal configurations are called isomers of each other.
Gamma radiation is very common after alpha or beta decay, as the earlier decay very often leaves the daughter nucleus in an excited isomeric state. Usually, this happens so quickly afterward that the gamma emission seems essentially coincident with the alpha or beta decay. However, there are some decays that can leave a nucleus in a long-lived isomer which can persist for some time under the usual behavior of radioactive materials. Often, an excited isomer will decay to another, albeit lower energy, excited isomer which can itself decay. This leads to a gamma cascade, where the nucleus emits many gamma rays as it decays (usually with most of them within so short a time period that they seem to be simultaneous).
Gamma rays are extremely penetrating compared to alpha and beta radiation. They can be mostly stopped by several centimeters of lead, or on the order of a meter of concrete, water, or biological material. However, unlike charged alpha and beta radiation, gamma rays are uncharged. Hence, they do not have a fixed range in matter like alpha or beta particles. Instead, the probabilistic nature of their capture results in a situation similar to radioactive decay, where a fixed fraction of the incident gamma rays will be attenuated by any given thickness of a particular material. This leads to attenuation, with the intensity falling off as the Beer-Lambert law. There are three main processes by which gamma rays are attenuated:
Photoabsorption
When a gamma ray encounters an atom, its electromagnetic field can accelerate an electron away from the rest of the atom, giving all of the gamma ray's energy to the ejected electron. This is called photoabsorption, and the process is the photoelectric effect. The high energy electron produced behaves in all respects like the energetic electrons from beta decay; the main difference is that the highly penetrating nature of the gamma rays can act to produce the photoelectrons deep inside of a person or object even if no radioactive contamination is there.
Photoabsorption is the most important form of gamma ray attenuation at low energies and for heavier elements.
Compton scatter
A gamma ray that encounters an electron can scatter off the electron, imparting some of its energy to the electron and leaving in a different direction. The resulting energetic electron, again, behaves identically to the electrons produced by beta decay but, again, can be produced deep inside of a person or object. Although the incident gamma ray flux is decreased by the Beer-Lambert law, you get a build-up of scattered gamma rays in your system so the total gamma flux does not strictly follow the Beer-Lambert relationship. The scattered gamma rays can then go on to further interact with the environment until they either escape or are stopped through photoabsorption or pair production (the latter of which, of course, also tends to produce additional gamma rays).
Compton scatter can be the dominant form of gamma ray attenuation at intermediate gamma ray energies and is more important for light elements than heavy elements.
Pair production
When a gamma ray has enough energy, it can produce an electron and its antimatter counterpart (a positron) out of empty vacuum when it interacts with the electric field of a nearby atomic nucleus. These electrons and positrons then go on to act like the electrons and positrons from beta radiation, including the production of annihilation gamma rays at the end of the positron's track.
Pair production is the most important method of attenuation at high gamma ray energies. Although it can occur for any gamma ray at more than the energy threshold for producing an electron-positron pair, it only becomes significant at several times this threshold.
Photo-nuclear interactions
It is possible for gamma rays to directly interact with a nucleus. This is not usually an issue for gamma rays produced by nuclear radioactivity, but it can be significant for some applications when considering very high energy gamma rays from more exotic processes.
One method of nuclear-gamma interaction is when a gamma ray excites an otherwise stable nucleus to one of its more energetic isomers, getting absorbed in the process. This requires a gamma ray of very nearly exactly the same energy as the isomeric transition. In most cases, the isomer is so short lived that it immediately decays, producing gamma radiation of nearly the same energy as the incident gamma ray going in a random direction. This is called nuclear resonance fluorescence, or NRF. However, a small portion of the energy of the interaction goes into the recoil of the absorbing and emitting nucleus, so that the re-radiated gamma ray no longer has the right energy to further participate in nuclear resonance fluorescence. NRF might be useful in the future for scanning materials for elements or isotopes of interest, but has little relevance to the off-resonance gamma rays emitted by nuclear radioactivity or positron annihilation or the broad spectrum Compton scatter gamma rays and this is generally of little significance.
At gamma ray energies well above that of most nuclear decays, in the range of 8 to 15 MeV, you can get a process where the electric field of the gamma pulls on the charged protons of a nucleus, tugging them all in one direction. The neutrons, being uncharged, are not pulled by the gamma ray's field and are left behind. The nuclear force of the neutrons then pulls the protons back. If the protons respond in about the same amount of time it takes for the fluctuating electric field of the gamma ray to change direction, the gamma ray will now be pushing on the protons in the same direction that the neutrons are tugging on them, causing them to overshoot so that they are again pulled back by the neutrons and pushed back by the gamma's fields. In the same way that small periodic pushes of a child on a playground swing at just the right time can build up a high amplitude motion, a gamma ray at this resonance energy can put all of its energy into an excited nuclear state of the protons sloshing around in the opposite direction of the neutrons, called a giant dipole resonance. Giant dipole resonances usually decay by ejecting nuclear particles – neutrons, protons, or light ions such as alpha particles, deuterons, tritons, or helions – although for very heavy atoms you can induce fission instead. This latter effect is called photofission.
For gamma ray energies above the giant dipole resonance, the chance of a photo-nuclear interaction goes down, but does not go away. Gammas with tens of MeV of energy or more can directly bump nuclear chips off of nuclei, or induce photofission.
Internal conversion
Instead of creating a gamma ray, it is occasionally possible for an isomer to decay to a less energetic isomer by giving up its excess energy to one of the electrons orbiting the nucleus. This is called internal conversion. The electron that receives the energy gets kicked out of the nucleus and, again, acts like a beta electron as far as material interactions. Internal conversion competes with gamma ray emission for transitions between isomers.
Gamma ray spectroscopy
Gamma radioactive decay produces gamma rays with very specific energies that depend on the decaying isotope. For example, the decay of the common long-lived fission product 137Cs nearly always produces a daughter product 137mBa in a metastable isomer with a half-life of 2.5 minutes. When the barium isomer decays, it always does so to the ground state isomer, making a 662 keV energy gamma ray. If you can detect an excess of 662 keV gamma rays, you'll know that there is 137Cs in the vicinity – possibly from nearby fission processes or un-accounted for radioactive material separated from spent fuel for medical or research purposes. Similarly, the isotope 60Co often results from neutron activation of metal alloys containing cobalt. When it decays to its daughter 60Ni, it nearly always does so to an isomer with an energy of 2506 keV above the ground state. This nearly always gamma decays to an isomer with 1333 keV of energy, releasing the energy difference as a 1173 keV gamma ray; the 1333 keV isomer then decays to the ground state releasing a 1333 keV gamma ray. This pair of 1173 keV and 1333 keV gamma rays are characteristic of 60Co.
Fortunately, it is not that difficult to detect the energy of a gamma ray.
Materials called scintillators produce a flash of light when exposed to ionizing radiation, and the amount of light they make depends on the amount of ionization caused inside of them, which in turn is proportional to the energy deposited by the gamma ray. So by measuring the light output of a scintillator crystal and comparing to callibration standards with known gamma ray energies (such as samples of the aforementioned 137Cs and 60Co), you can get a reasonably good idea of the energy of the gamma rays interacting with your scintillator. Scintillators provide rather broad energy peaks, so you can't always pin down the energy all that well, and some sources may be obscured by other, stronger sources with similar energy gamma rays, but scintillators tend to be relatively cheap and convenient to use (although the crystals are not always robust and most are hygroscopic - if not sealed they absorb water vapor from the atmosphere and become ruined). Some varieties of scintillator (like LaBr3) offer better resolution than more common and less expensive varieties (like the old workhorse NaI). Some scintillators are valued because they can discriminate between gamma ray events and neutron or ion signals by comparing the rise time and falloff time of the light pulses.
For better energy resolution, you can go to semiconductors. Here, a sample of high purity semiconductor is put under a high electric field. When the semiconductor absorbs radiation, the electrons drift one way in the field, and the holes (missing electrons) the other way. This creates a current pulse, which can be detected by the electronics. Without the additional light emission step, and with better statistics from the (usually) larger amount of ionization, semiconductors can more precisely pin down the energy of gammas they are exposed to. The gold standard among radiation detectors are high purity germanium detectors (HPGe). These provide exceptional energy resolution, although they need to be cryogenically cooled to around liquid nitrogen temperatures in order to work. While modern HPGe detectors can be electrically cooled, alleviating the need for bulky liquid nitrogen coolers, starting one up in the field can still take tens of minutes for the germanium crystal to cool down.
Superconducting bolometers provide even better resolution than the vaunted HPGe detectors. They are, however, large, bulky, and require refrigeration to liquid helium temperatures. As a consequence, they are mainly only used as fixed instruments for science experiments.
A gamma spectrum of a radiation source will help an investigator determine what isotopes or other physical processes are present (such as fission or bremsstrahlung) that are producing the gamma rays. This in turn can be useful for a wide range of applications, from nuclear forensics to prospecting for ores.
Neutron
Neutrons are not usually emitted by radioactive decay (although some very short lived isotopes that undergo beta decay can also emit a neutron in the process). However, neutrons are emitted in copious amounts by fission and (especially) fusion reactions. They are also produced by various high energy processes that can knock off bits of atomic nuclei or even make the nuclei disintegrate into fragments, such as high energy particle beams or antimatter annihilation.
Neutrons make for very penetrating radiation, on the same order of penetration as gamma rays. However, while gamma rays are best stopped by heavy elements the best ways to stop neutrons is with light elements. Neutrons, being uncharged, do not leave behind ionization tracks. Instead, they lose their energy by interacting directly with nuclei.
Elastic collisions
The most straightforward kind of interaction is one where the neutron simply strikes and then bounces off a nucleus[1]. The struck nucleus will recoil when the neutron rebounds, and the energy of the original neutron is now distributed between the recoil energy of the nucleus and the kinetic energy of the scattered neutron. The closer in mass the nucleus is to the neutron, on average the more of the neutron's energy will be transferred to the nucleus. For a neutron bouncing off an individual proton with nearly the same mass, the effect is analogous to billiard balls striking each other with, on average, the neutron losing half of its energy to the proton with each bounce. For heavier nuclei, the effect would be more analogous to a billiard ball striking a bowling ball, with the billiard ball rebounding with most of its original energy and little energy going into the bowling ball. Thus hydrogen (with its nucleus of just a single proton) is the best shielding material against energetic neutrons. It will usually take repeated collisions for a fast neutron to be slowed down to the point that it can be absorbed.
The recoil nucleus becomes an energetic ion, and thus will leave behind a dense ionization track that is particularly effective at causing biological damage.
Neutron capture
Sometimes, when a neutron hits a nucleus, instead of bouncing off it might stick[1]. This adds the neutron's energy to that of the new nucleus, giving an excited isomer that usually decays by producing a gamma ray. Occasionally, however, the isomer can decay by spitting off charged particles instead. Neutron capture is more likely for lower energy neutrons, so high energy neutrons entering a material may need to bounce around for many collisions before they are slow enough to be absorbed in this fashion.
Nearly any isotope can absorb a free neutron, but some isotopes are better at this than others. Some are much better. 157Gd has the best neutron capture cross section of any stable isotope, and 155Gd is also a spectacularly good neutron capture isotope. So spiking a material with gadolinium can help it sop up excess neutrons. Similarly, 10B, 6Li, and 113Cd have an exceptionally high neutron capture cross sections. The former two even lose their capture energy by emitting alpha particles (although 10B also emits a gamma ray) so pose less of a gamma hazard.
In any event, neutrons present in a material will produce gamma rays, so once the neutrons are stopped you will then need to worry about the gammas.
Inelastic collisions
If a neutron slams into a nucleus hard enough, it can knock off bits of it. These bits can be other neutrons, protons, deuterons, tritons, helions, alpha particles, or occasionally even heavier nuclei. It can also happen that the neutron collision just excites the nucleus to a higher energy isomer before it bounces off, which will gamma decay. All of these inelastic collisions take away more energy from the incident neutron than elastic collisions where the neutron just bounces off while leaving the nucleus in its original state. Thus, for very high energy neutrons you can get by with fewer collisions to bring the neutron down to energies low enough for capture than you would by elastic collisions alone. At the price, of course, of producing additional radiation you need to shield against.
Fission
When a neutron hits a fissionable nucleus, it can impart enough energy to lead to fission. For fissile nuclei, fission can occur directly from the energy imparted by neutron capture.
Activation
When a neutron is captured, the new nucleus might be unstable and these unstable nuclei usually decay by beta decay. Thus, neutron radiation makes its surroundings radioactive. This is called activation.
Neutron energy
Neutrons are often characterized by their energy. Those released by nuclear processes initially have enough energy that they are classified as fast neutrons. Fast neutrons scatter via elastic and inelastic collisions until they lose enough energy to fall below the inelastic thresholds. They then enter an epithermal energy regime. Epithermal neutrons are still moving fast enough that neutron capture is unlikely, so they go through the long, slow (for atomic scale processes, still instant on human time scales) process of losing energy by elastic scatters. Finally, the neutrons lose so much energy that they have nearly the same kinetic energy as the nuclei around them. These are called thermal neutrons, and they can no longer lose energy (on average) by colliding with the nuclei of the material. It is thermal energy neutrons that are most susceptible to neutron capture and removal from the system.
Radiactive decay
Neutron radiation is almost always quickly caught by some nucleus in the environment after it scatters around for a bit. But for cases where that is not possible (like in outer space), it is worthwhile to note that free neutrons themselves are radioactive, decaying into a proton, electron (beta particle), and electron anti-neutrino with a 15 minute half-life.
X-rays and Auger electrons
After undergoing radioactive decay, the resulting daughter atom will have a different relaxed electronic structure than the original parent atom. Because the decay happens so fast that the electrons cannot follow along with the changes, the daughter is often left in an excited electronic state, with the electrons out of order and some electrons missing from the core orbitals. Less tightly bound electrons can fall into the empty core holes, but this liberates energy which must go somewhere. That energy can be shed by one of two processes. The atom can emit a photon, called an x-ray, which behaves like a somewhat lower energy gamma ray. Or the in-falling electron can give up its energy to one of the outer, less tightly bound electrons which is then kicked out of the atom and acts like a low energy beta ray. This last process is called the Auger process, and is more likely for light elements while x-ray radiation is more likely for heavy atoms.
Muon
Muons are particles that behave like heavy electrons. They are far too energetic to be produced by nuclear radioactive decay, but they are commonly produced by the decays of some sub-atomic particles and in the collisions of particles created by particle accelerators or from cosmic rays. In particular, they are a common form of natural background radiation on earth coming from the cosmic rays bombarding our atmosphere from space. Muons are extremely penetrating, leaving sparse ionization trails like electrons but with far more mass giving them more momentum and more energy to shed. It can take hundreds of meters of rock to screen out most of the muons created by cosmic radiation that manage to reach ground level.
Exotic particles
The decays of excited hadrons and fundamental particles can produce a whole zoo of crazy exotic particle radiation. Usually the only kinds that can get very far are charged pi mesons (pions) and both neutral and charged K mesons (kaons), which can often travel several meters before decaying into muons or (for kaons) into pions; and stable anti-particles such as positrons and anti-protons or anti-neutrons.
The mesons will be stopped in matter much like muons, except that they can also participate in nuclear collisions and thus will likely be stopped after passing through about a meter of condensed matter by getting absorbed by an atomic nucleus. When this happens, the particle can decay almost immediately by breaking the nucleus apart into fragments, namely neutrons and light to medium weight ions. Negatively charged pions and kaons that are stopped in matter will be attracted toward positively charged nuclei, so that they will usually be absorbed by the nucleus before they can decay on their own leading again to nuclear disintegration.
Positron stopping and reactions have already been discussed. Anti-protons and anti-neutrons will be stopped like normal protons and neutrons except that if they hit a nucleus they will annihilate one of the protons or neutrons in the nucleus to produce several (on average three) very energetic pions (or occasionally kaons) The neutral pions almost immediately decay into very high energy gamma rays, the others behave as described above.
Effects of radiation
Dose
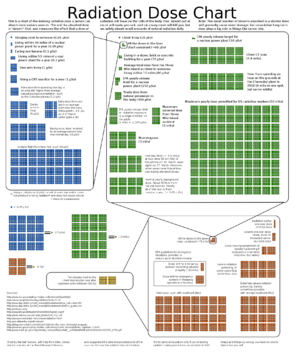
The amount of radiation exposure is generally quantified by the amount of energy deposited by radiation in a given amount of material (such as body tissue), called dose. The SI unit of dose is the gray (Gy), defined as 1 joule of deposited energy per kilogram of material. To measure chronic biological effects, it is often necessary to weight the dose by a factor that depends on the kind of radiation – for example, alpha particles cause more severe health problems than beta or gamma particles for a given dose. This is the effective dose or equivalent dose, and is measured in seiverts (Sv). A whole Gy or Sv is a very large dose, so it is often convenient to use milligrays (mGy) or milliseiverts (mSv) for measuring chronic, low dose, or background exposures. The conversion is 1000 mGy = 1 Gy and 1000 mSv = 1 Sv.
Other measures of dose are sometimes used. Older sources may refer to rads, with 100 rad = 1 Gy, or rem, with 100 rem = 1 Sv. A more humorous unit is the BED, or Banana Equivalent Dose – the amount of dose you get from eating one banana. 1 BED is often taken as 0.1 μSv.
Biological effects
The ionization produced by energetic radiation liberates many electrons and leaves behind a plethora of broken chemical bonds caused by missing electrons (often called holes). The free electrons can lose energy by producing more ionization or by exciting vibrations until they are low enough energy to stick to molecules; while any deep core holes can decay via the Auger process or emission of x-rays to migrate to the valence electrons and produce molecules lacking an electron. These molecules without the right number of electrons are called free radicals, and they are very chemically reactive. Inside the body, they will readily attack the bio-molecules needed for life. Depending on the amount of ionization a cell is exposed to, it may recover without issue, it might recover but remain in a long-term damaged state, or it might die. Rapidly dividing cells are the most susceptible to damage from these free radicals.
Acute
High levels of radiation dose leads to many cell deaths. In humans and other mammals, this usually requires at least 0.7 Gy delivered within several minutes. As this affects rapidly dividing cells at lower doses than more quiescent cells, people exposed to high levels of radiation experience symptoms from their rapidly dividing cells being killed. This includes nausea from the cells lining the gut being destroyed, as well as life-threatening issues due to the loss of blood cells – anemia from the lack of red blood cells causing fatigue and weakness, hemophilia from the lack of platelets causing uncontrolled bleeding, and a non-functional immune system due to the lack of white blood cells allowing infections to run rampant in the body. In addition, a patient's hair often falls out (with 3 Gy or more) and they may experience skin damage in the form of reddening, irritation, itching, blistering, and ulceration[4].
Doses of less that 2 Gy are usually survivable. Doses over 6 Gy are almost always lethal without medical care, and over 8 Gy are almost always lethal even with medical care.
At even higher doses (> 30 Gy), the radiation can simply shut down the victim's nervous system, causing rapid death.
Because they are still growing and acute radiation syndrome attacks rapidly dividing cells, children, infants, and fetuses are especially sensitive.
Chronic
Cells which are damaged but do not die may remain in a damaged state for some time. This increases the risk that a damaged cell may malfunction when it has to reproduce, leading to a situation where the cell begins to proliferate out of control. In this way, radiation dose can lead to an overall increase in lifetime risk of cancer. It is estimated that cancer risk increases at 5.5% per Sv of effective dose, although the validity of this linear model is questionable at low doses near background levels where threshold effects may come in to play.
Mutations
If damage occurs to reproductive cells (sperm or eggs), it can cause changes to the genome that can be transmitted to future offspring. Many of these changes will simply be fatal to the developing embryo or fetus. Others lead to various genetic disorders that can decrease overall health of the child. Occasionally, it may lead to a change that improves the offspring's ability to adapt and thrive in a particular environment – although this latter effect has not been demonstrated in humans, it is regularly observed among plants, insects, and microbes.
Background radiation
The natural environment we live in has many sources of radiation, to which we are continually exposed[5].
Cosmic radiation is the radiation that comes from space. In space, this is typically high energy protons and light ions. When these particles hit Earth, they interact in the upper atmosphere to produce radiation showers. Nearly all of this is attenuated by Earth's thick atmosphere before it hits the ground, but a small fraction will reach ground level and deliver dose to our bodies. Higher altitudes provide less air to stop cosmic ray showers and less distance in which any muons in the showers can decay, so living at high altitude or going on an airplane flight exposes you to increased cosmic background radiation. For example, a coast-to-coast commercial airline flight across the United States will increase a passenger's exposure by about 0.01 mSv.
Naturally occurring radioactive material (NORM) is radioactive isotopes that are found in nature. The four isotopes that make up almost all NORM are 238U, 235U, 232Th, and 40K, as well as the isotopes in the decay chains of 238U, 235U, and 232Th (40K decays directly into stable 40Ar, so it has no other radioactive isotopes in its decay chain). The amount of NORM in the environment depends on the local geology, with some rocks rich in potassium and actinide elements (like granite) providing more background radiation than others. One of the isotopes in the 238U decay chain is 222Rn, a gas that is present in the air with a 3.8 day half-life. Breathing this radioactive gas, drinking dissolved uranium and thorium in water, and exposure to the gamma rays produced by decaying NORM all contribute to our background dose.
We even have radiation exposure from sources inside our own bodies. Potassium is an element vital for life, and some small portion of that is 40K. This is present in all biological material; in the food we eat, in our bones and blood and muscles. As it decays, we are exposed to its radiation. Married people have a slightly higher background dose rate than single people, because they sleep next to their spouse who is also a source of radiation from 40K decays. To a lesser extent, 14C also provides an internal dose. 14C is made by cosmic ray bombardment of oxygen and nitrogen high in the atmosphere. This then forms CO2 which is taken up by plants to form their tissues, which then in turn are eaten by animals and used to form the animal tissue. And thus all the food we eat and all the tissues in our bodies also contain 14C which gives a small contribution to our internal dose.
Different people in different environments receive different background radiation dose rates, but in the United States 3 mSv/year is typical.
Threshold dose
Cells can repair themselves after exposure to radiation. As living organisms, we appear to be well adapted to repair radiation damage at dose rates typical of our natural background radiation. Even at dose rates well above that for typical background levels (for example, for people living at high altitudes where there is less atmospheric protection from cosmic rays or for people living over bodies of radioactive ores or other naturally radioactive rock – particularly granite. Or for astronauts, flight crew on commercial aircraft, or people given medical procedures resulting in exposure to low dose radiation such as CAT scans or x-rays), there is no detectable increase in cancers or mutations. There appears to be some threshold above which the dose or dose rate can cause harm and below which it is harmless; although quite what this threshold dose rate is has not yet been teased out from the data.
Government agencies responsible for worker safety generally establish allowable regulatory dose limits; both for the general public and for radiation workers[6]. This varies from country to country, but is usually set at a level thought to be well below the threshold of significant risk. While there are many specifics dealing with exposures to various body parts, as an example the allowable whole body dose set by the Nuclear Regulatory Commission (NRC) of the United States of America is 1 mSv/year for a member of the general public and 50 mSv/year for radiation workers above and beyond any background radiation, with a lifetime allowance of 250 mSv (this only applies to dose received at facilities under NRC jurisdiction, so it will not cover, for example, medical imaging or exposure at foreign sites). In the United States, at least, these values are usually set conservatively with the goal of avoiding harm. Individual institutions under the authority of U.S. law then often set even more stringent limits for their workers to avoid falling foul of federal regulations.
Material effects
Heavy neutron or ion bombardment can have dramatic effects on materials. The nuclear recoil from collisions knocks atoms out of place. This will produce crystal defects, make materials amorphous, and cause materials to become brittle. In addition, neutron capture transmutes the material into other isotopes. These can change the chemical composition of the material, potentially weakening it. And it also makes the material radioactive, generally decaying via beta decay to produce beta and gamma radiation.
the amount of radiation needed to affect material changes depends strongly on the material. But for example, reactor steels experience embrittlement at neutron fluences of on the order of 1019 to 1020 fast neutrons (E > 1 MeV) per square centimeter. This corresponds to a neutron dose on the order of between 10,000,000 and 1,000,000,000 Gy. Naturally, reactor steels are chosen for their resistance to radiation so more typical materials may be more sensitive. But this is still many orders of magnitude less sensitive than biological tissue.
The effects of gamma and beta radiation on materials are more subtle. They can activate F-center defects, causing transparent materials to change color and lose transparency. The ionization they create can also lower activation barriers to chemical reactions, directly break chemical bonds, and create free radicals that chemically attack the material, causing rubber and plastics to crack and become less bendable and more brittle, bleaching organics, and eventually causing organic materials to degrade. The lower activation barriers can also help to anneal disordered materials back into a crystalline lattice.
Electronics effects
Modern electronics use tiny structures etched in silicon that can be triggered by tiny currents. The ionization pulse from radiation interaction can easily flip a bit that was not meant to be flipped, cause transient stray currents that interrupt operations, produce low impedance shorts that result in continued unwanted current draw (latch-up), or even produce a high enough current to permanently freeze a bit or destroy power electronics. The results range from momentary glitches in operation, faults that last until the device is powered down and restarted, or permanent damage to the device[7][8]. Ions (including alpha particles and protons) and neutrons are more likely to produce worse effects due to their higher localized ionization density.
In addition to these single event effects, the total ionizing dose an electronic device receives can affect its performance from effects such as threshold shifts, increased leakage current and power consumption, and timing changes. With enough total ionizing dose, the device may cease to function properly, or at all[9][10].
Ions (including alpha particles) and neutrons can displace atoms in the electronics' structure. This can alter the electrical properties of the affected region. This can result in permanent damage to the electronic device[11][10].
The tolerance of an individual electronic component to radiation exposure can vary wildly depending on the device, hardening methods, and shielding. A typical lifetime dose for space radiation equipment is often around 300 to 1000 Gy[10], so space-rated electronics are often designed to handle these doses. Radiation-hardened electronics can be made to survive up to 3000 Gy [12]. Consumer grade electronics can be expected to fail at significantly lower doses, at 50 to 100 Gy.
Credit
Author: Luke Campbell
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Kenneth S. Krane, "Introductory Nuclear Physics", John Wiley & Sons, New York (1988)
- ↑ National Nuclear Data Center Chart of Nuclides
- ↑ XKCD radiation Dose Chart
- ↑ Acute Radiation Syndrome: A Fact Sheet for Clinicians (CDC)
- ↑ U.S. NRC, Background radiation
- ↑ The DOE Ionizing Radiation Dose Ranges Chart
- ↑ A Quick Overview of Radiation Effects – Single Event Effects
- ↑ Single Event Effects
- ↑ Total Ionizing Dose (TID) Effects
- ↑ 10.0 10.1 10.2 A Quick Overview of Radiation Effects
- ↑ Displacement Damage
- ↑ ATMEL Rad hard 16 MegaBit 3.3V SRAM Multi-Chip Module AT68166H product data sheet